DATA AND ANALYSIS
Boltzmann's distribution equation, P(state)=(constant)e^((-E)⁄kT), gives the probability distribution where E is the energy in a state, k is Boltzmann's constant, and T is the temperature. For a solution, this equation can be rearranged so the slope E of ln(x) vs. 1/kT is the enthalpy of dissolution.
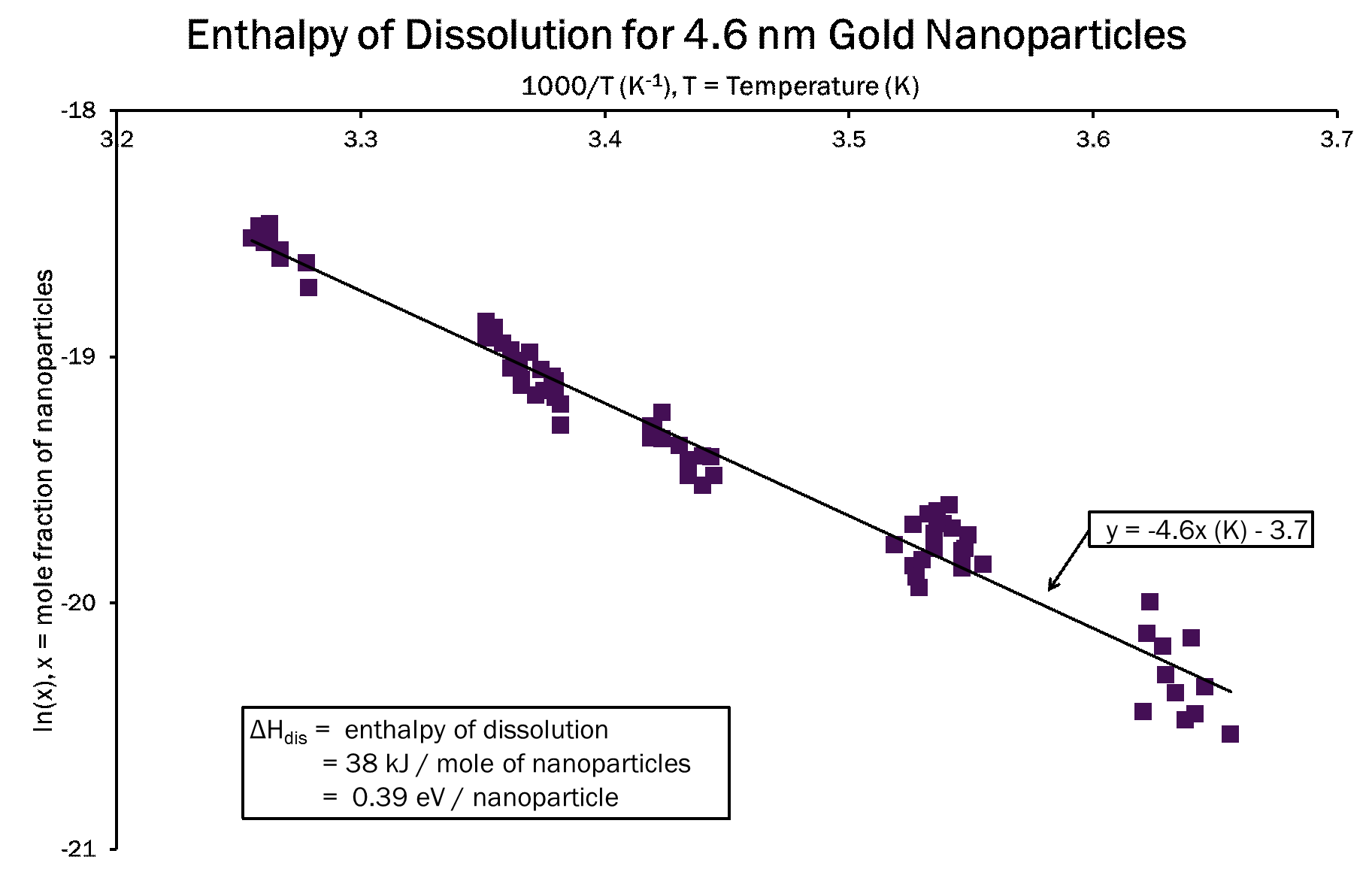
The melting temperature of the nanoparticle superlattice is estimated using the enthalpy of dissolution. The melting temperature of the nanoparticle superlattice is calculated assuming that it behaves similar to an inert gas solid.The enthalpy of dissolution is related to the depth of the potential well between two interacting particles.The depth of the potential well is used to calculate the melting temperature of the superlattice. For an ideal solution, the ln(x) will equal zero at the melting temperature of the nanoparticle superlattice. If the solution is not ideal, ln(x) + ln(γ) = 0 at the melting temperature.

FUTURE PLANS
The enthalpy of dissolution for our gold nanoparticles ligated with DDT and suspended in toluene is 38 kJ/mole of nanoparticles or 0.39 eV/nanoparticle. Our data is limited to a relatively small range of temperatures. A stronger binding ligand will give a more accurate enthalpy of dissolution because data can be acquired over a larger range of temperatures without the nanoparticle sample breaking down.
The activity coefficient of the nanoparticles in solution with 35% DDT is 280. Better estimates of the nanoparticle superlattice melting temperature will produce a more accurate activity coefficient.
Reproduction of these results with different samples of nanoparticles is ongoing. Dependence of nanoparticle interactions on factors such as particle size, shape, metal, ligand, and solvent can be studied in the future. Understanding nanoparticle interactions is important if we plan to implement them in technology.



