EXPERIMENTAL PROCEDURE
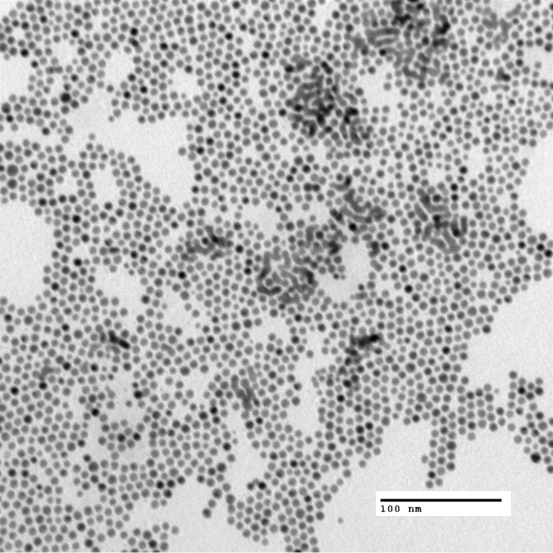
Our 4.6 nm, spherical gold nanoparticles are synthesized by the inverse mecelle method, ligated with dodecanethiol (DDT), and dissolved in toluene. The image below is taken from a transmitting electron microscope (TEM). All of the dark circles are our spherical nanoparticles which should be approximately uniform in shape and size due to digestive ripening. The nanoparticles are coated with a ligand (DDT) which binds to the gold and prevents the nanoparticles from breaking down.
Thermal energy suspends some nanoparticles in solution. The rest of the nanoparticles form aggragates and fall. The aggragates fall faster than the monomers suspended in solution because they have a larger mass. Since thermal energy causes the suspensions, we can measure the relationship between the concentration of suspended nanoparticles and temperature.
The sample reaches two-phase equilibrium in a temperature controlled centrifuge. In this case, two-phase equilibrium implies that our solution has both suspended and precipitate nanoparticles. For the system to be in equilibrium, the concentration of the nanoparticles suspended stays constant. The number of nanoparticles precipitating out will be equivalent to the number dissolving into the solvent. We allow 15 minutes for the sample to reach equilibrium.
After the sample is in two-phase equilibrium we spin the sample in the centrifuge for 5 minutes at a force of 3,300 g. Below are two pictures of our centrifuge. The image on the left is our rotor with the sample inserted. The image on the right shows a bird's-eye view of the centrifuge. The copper coils are connected to two temperature controlled circulating baths. These pump a 50/50 mixture of antifreeze and water through the coils to change the temperature of the centrifuge.
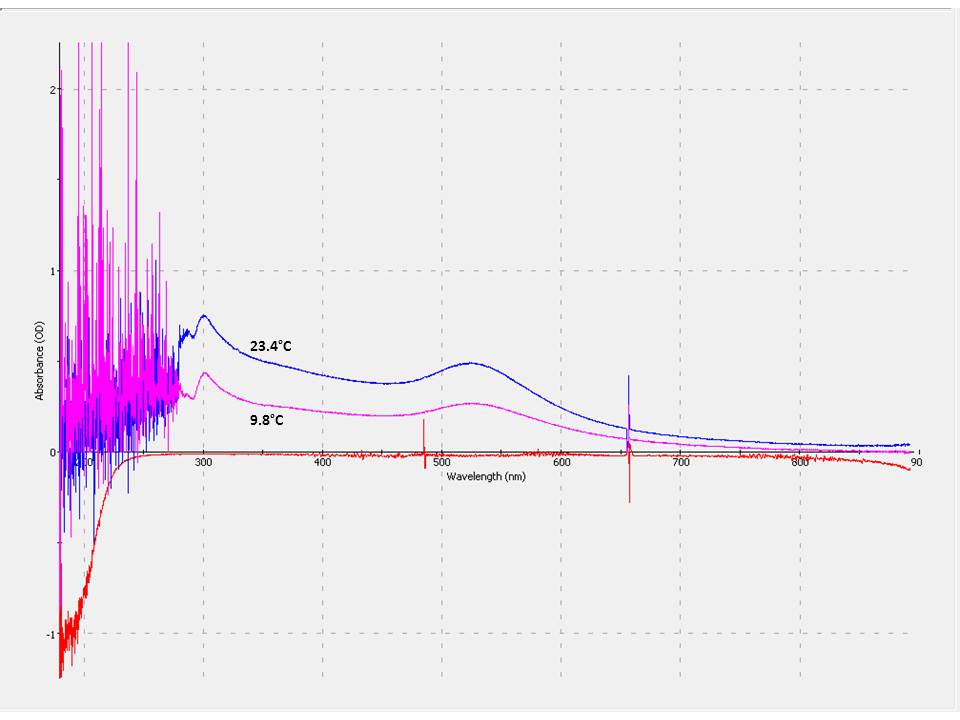
UV-Vis spectroscopy through the supernatant is used to measure the concentration of nanoparticles using absorbance of the nanoparticles using absorbance of the nanoparticles from 200-900 nm. For our 4.6 nm gold nanoparticles, the plasmon peak is at 524 nm. As the temperature of the system increases we see an increase in absorbance because more nanoparticles are suspended in solution. The spectra below shows absorbances dependence on temperature. Using the absorbance at the plasmon peak, we can calculate the concentration and mole fraction of nanoparticles in solution.