Imaging Molecular Dynamics with a Time-Stamping Camera
Paige Lettow, Hillsdale College, Physics and Mathematics Major
Mentored by Dr. Vinod Kumarappan
The main idea of this research project is to study the rotation of individual molecules on super small timescales. We want to “take a picture” of a molecule rotating at a single moment in time; however, the rotation of molecules happens on the order of picoseconds, which is far too fast for any electronics to capture. Instead of using electronics to capture this image, my research group used lasers in a pump-probe experiment.
The pump-probe experiment (Fig. 1) consists of a single laser that is split into two beams. Each beam takes a different path to our molecule, creating a small time difference between the two. Both beams are sent to a vacuum chamber where the sample molecule is being pumped in. We use the vacuum chamber to ensure that we are looking only at individual molecules from our sample. The pump beam comes into contact with our sample first, aligning the molecules and kicking the molecules into rotation. Then, the probe beam hits the sample and breaks the molecules apart. All this happens inside a velocity map imaging spectrometer (not shown in the figure) in which electrostatic fields push the ions toward the detector. When the molecule is broken apart, the fragments fly toward the sensor (a microchannel plate detector with a phosphor anode) and the signal is picked up by our camera. We collect information about when and where each fragment hit the sensor, and from that information, we can work backwards to calculate what the molecule looked like before it was split apart.
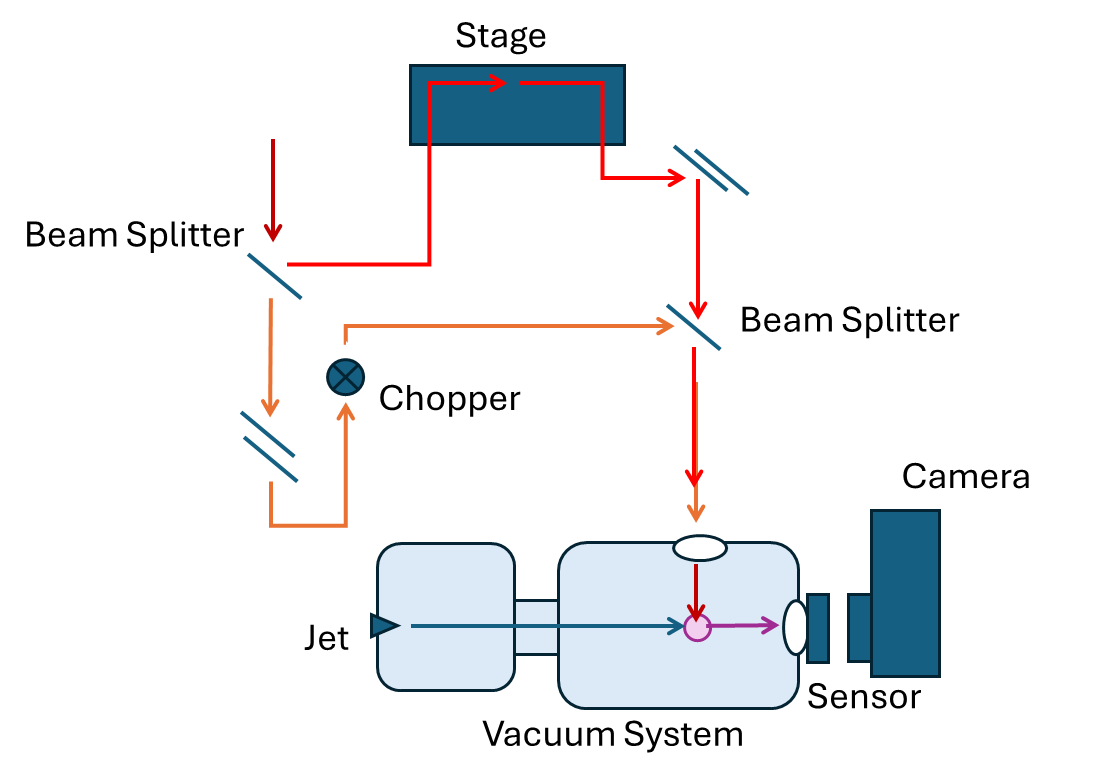
Fig. 1. A schematic of the experimental setup.
The molecule my group studied was epichlorohydrin. When the pump beam hits the molecules, they all align in the direction of the laser polarization and begin rotating in two ways. They mainly rotate lengthwise (Fig. 2 A), but because epichlorohydrin does not have a symmetry axis, the molecules also rotate around the z-axis (Fig. 2 B). While our data shows the first type of rotation clearly, the second kind of rotation is what we are more interested in.
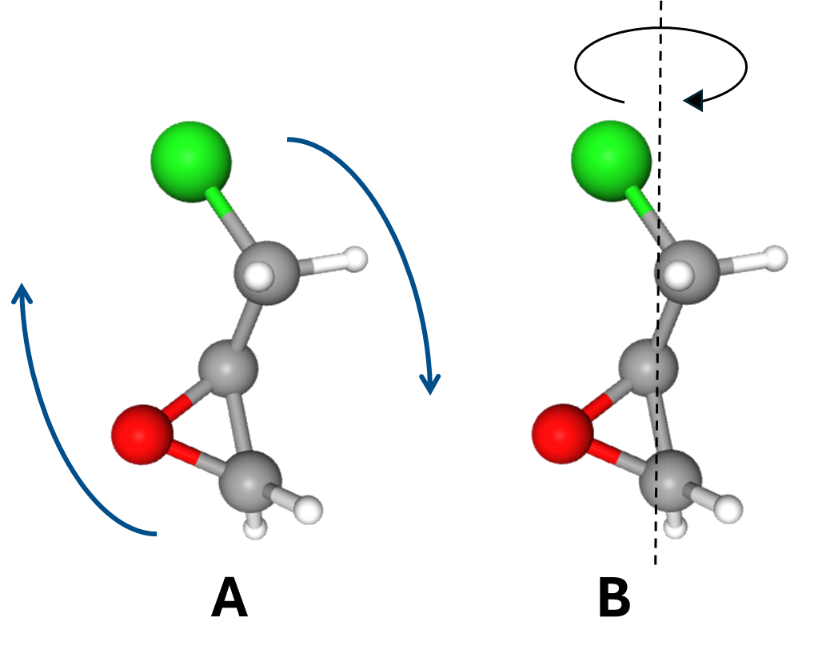
Fig. 2. The two types of rotation for epichlorohydrin. Molecule image adapted from https://pubchem.ncbi.nlm.nih.gov/compound/16213567#section=3D-Conformer.
All of our data collection was done using the TPX3 Camera [1]. This camera has over 65,000 pixels, and each pixel collects information on the Time-of-Arrival (ToA), the time between the shutter opening and receiving the signal, and Time-over-Threshold (ToT), how much signal was collected. The data from the TimePix Camera comes as a series of values containing x and y position (which pixel the data is from), ToA, ToT, and a universal time stamp telling us in what order each signal was received.
When the molecule is broken apart into fragments, those fragments hit the sensor at different times. For my experiment, each fragment was singly charged, so the lighter fragments moved faster than the heavier fragments and hit the sensor first. In Figure 3 there is a Time-of-Flight (ToF) graph showing the number of hits received at each time. We can determine which peak corresponds to which fragment because, with all singly charge particles, ToF is proportional to mass over charge. For example, the first peak in Figure 3 is hydrogen because it is the lightest of the particles.
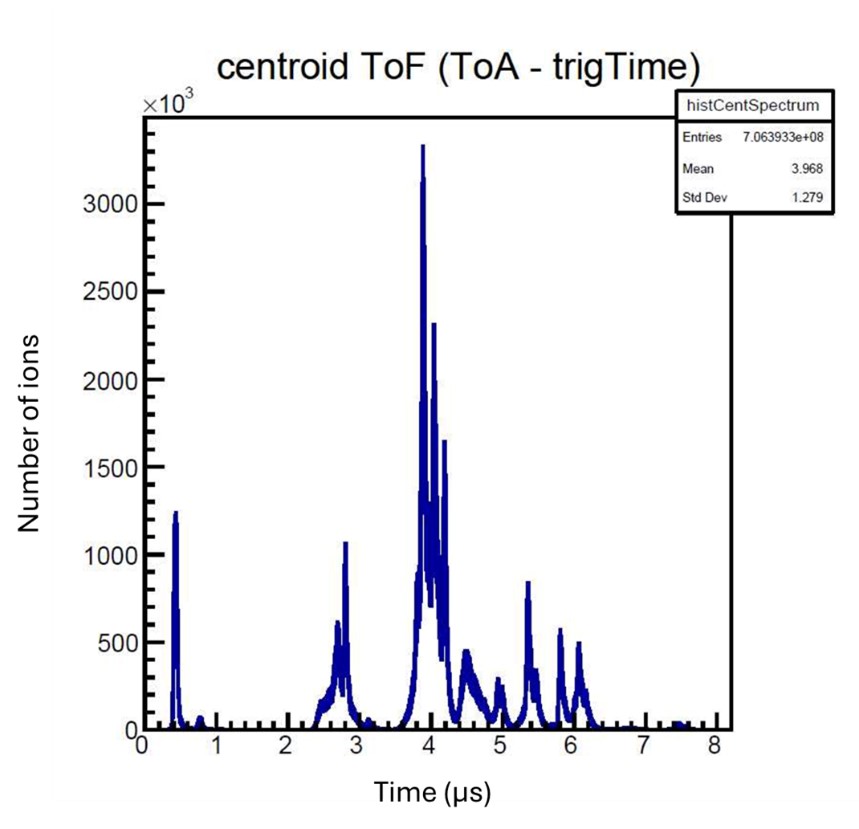
Fig. 3. ToF graph when the pump beam is at 60mW and the probe is at 74mW. The centroid graph looks at the clumps of pixels where a single fragment hit the sensor to determine the center of the hit and the best pixel to collect data from.
We have collected a lot of data in which the first kind of rotation (Fig. 2 A) can be seen, using various powers for the pump and probe beams. Figure 4 shows this rotation. The tall spike at the beginning of the graph is due to pump-probe overlap; the signal shoots up here due to constructive interference of the two beams. The signal immediately after this is due to the alignment of the epichlorohydrin molecules. The molecules start off aligned, giving us our original signal, and then they rotate at different frequencies, eventually coming back to the original alignment in a full revival. A revival of the signal can be seen in the graph at about 120 ps. This data that we obtained should contain the second kind of rotation as well, but it has not yet been fully analyzed.
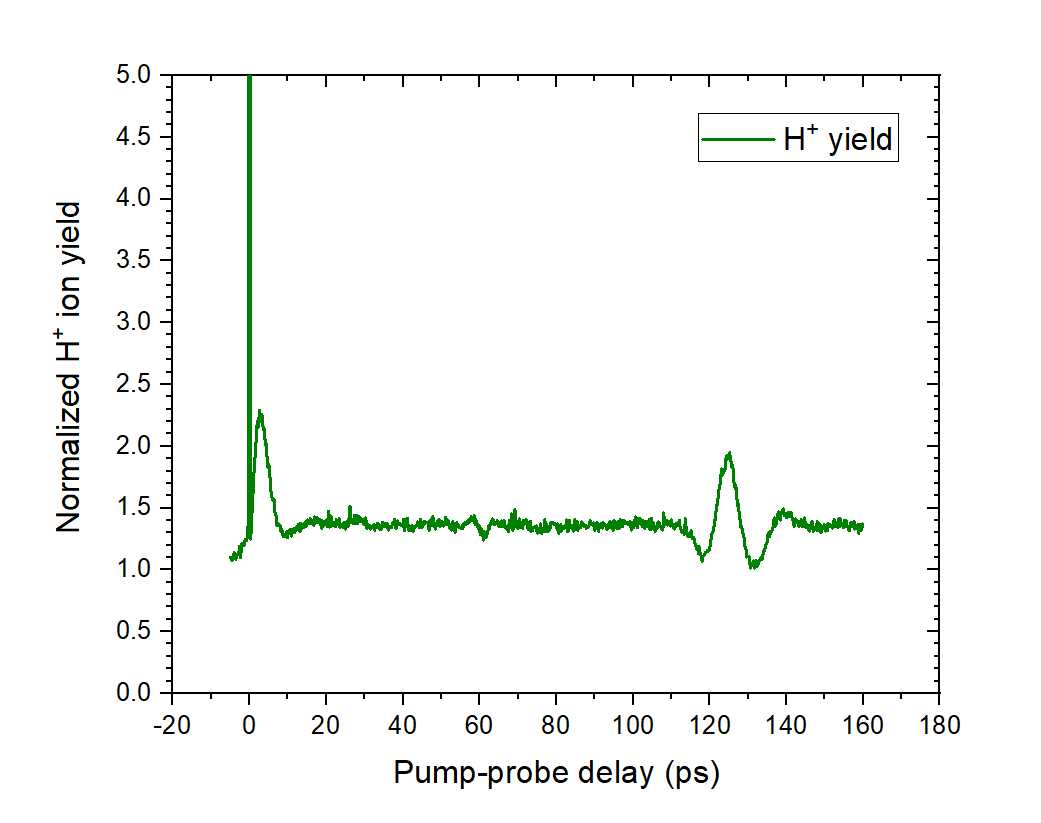
Fig. 4. Graph of the signal received from the hydrogen fragment.
For this project, I did a lot of work in the lab with lining up the components of the vacuum chamber in order to get a straight stream of molecules coming from the jet. The jet stream and both laser beams had to line up to hit each other in the middle of the vacuum chamber, so I lined up the optics to send the pump and probe beams perfectly into the chamber, making sure that the two beam paths were overlapped. Additionally, I did a lot of work with the setup and installation of the TimePix camera and the software we used alongside it. I have been running analysis code to compile the data and produce graphs, hoping to find that second, more subtle, rotational motion. In the future, this research group will continue to analyze the data that we have collected and eventually run a similar experiment with new molecules.
References
[1] Amsterdam Scientific Instruments https://www.amscins.com/
Acknowledgements
I would like to thank Dr. Vinod Kumarappan, my mentor for this project; as well as Dr. Varun Makhija, Dr Bob Jones, Dr. Eric Wells, Lana Chaleunrath-Pham, and Diksha. Thank you to Dr. Loren Greenman, Dr. Bret Flanders, and Kim Coy for putting together this research experience. This material is based upon work supported by the National Science Foundation under Grant Nos. 2244539 (the REU program) and 2018286 (the TPX3 camera). Participants from K-State were supported by the US Department of Energy under Grant No. DE-FG02-86ER13491. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Department of Energy.
Final Presentation