Two-Carbon Hydrocarbons in Strong Laser Fields
Sydney Leeper, Kansas Wesleyan University, Biomedical Chemistry and Physics Major
Mentored by Dr. Cosmin Blaga
Main Objective
Interaction of ultrashort femtosecond pulses (1 fs = 10-15 s) with matter has been extensively studied as it provides insight into ultrafast molecular processes. In this project we conducted a systematic investigation into the photoionization of two carbon-hydrocarbons to understand how the number of C-H bonds affects the molecular dynamic initiated by the 1030 nm, 450 fs laser pulses near the saturation intensities. We recorded mass and photoelectron spectra as a function as laser intensity, as well as photoelectron angular distributions at select intensities in ethane (C2H6), ethylene (C2H4), and acetylene (C2H2). Our study revealed common features in the three compounds as well as significant differences between the three compounds.
Background
When matter interacts with strong laser fields, energy from the laser pulse is deposited into the atom or molecule’s internal degrees of freedom, leading to photoexcitation, photoionization and/or fragmentation. Historically, these phenomena were investigated with photoelectron spectroscopy, i.e. the measurement of the liberated electron’s energy as a function of ejected angle with respect to the laser polarization, as well as mass spectroscopy, i.e. the analysis of fragmentation yields as a function of mass over charge ratios. For example, photoelectron spectra demonstrated that liberated photoelectrons have a small probability to revisit the parent ion where it can be recaptured or undergo elastic or inelastic scattering. This rescattering mechanism lies at the heart of high harmonic generation, laser induced electron diffraction and non-sequential ionization, respectively. Since molecular structure determines the physical and chemical properties of matter, laser induced electron diffraction, when performed with ultrashort pulses, allows to determine bond lengths and angles as molecules undergo a chemical reaction. Therefore, collecting photoelectron angular distributions (PAD) can allow us to study ultrafast molecular dynamics. In our study we focused our attention on ethane (C2H6), ethylene (C2H4), and acetylene (C2H2) when subjected to intense 1030 nm lasers. At this wavelength, the interaction is non-resonant with the vibrational modes. In the future, we plan to retake this study with mid-infrared pulses resonant with the C-H stretch mode centered at 3300 nm.
Experimental Methods
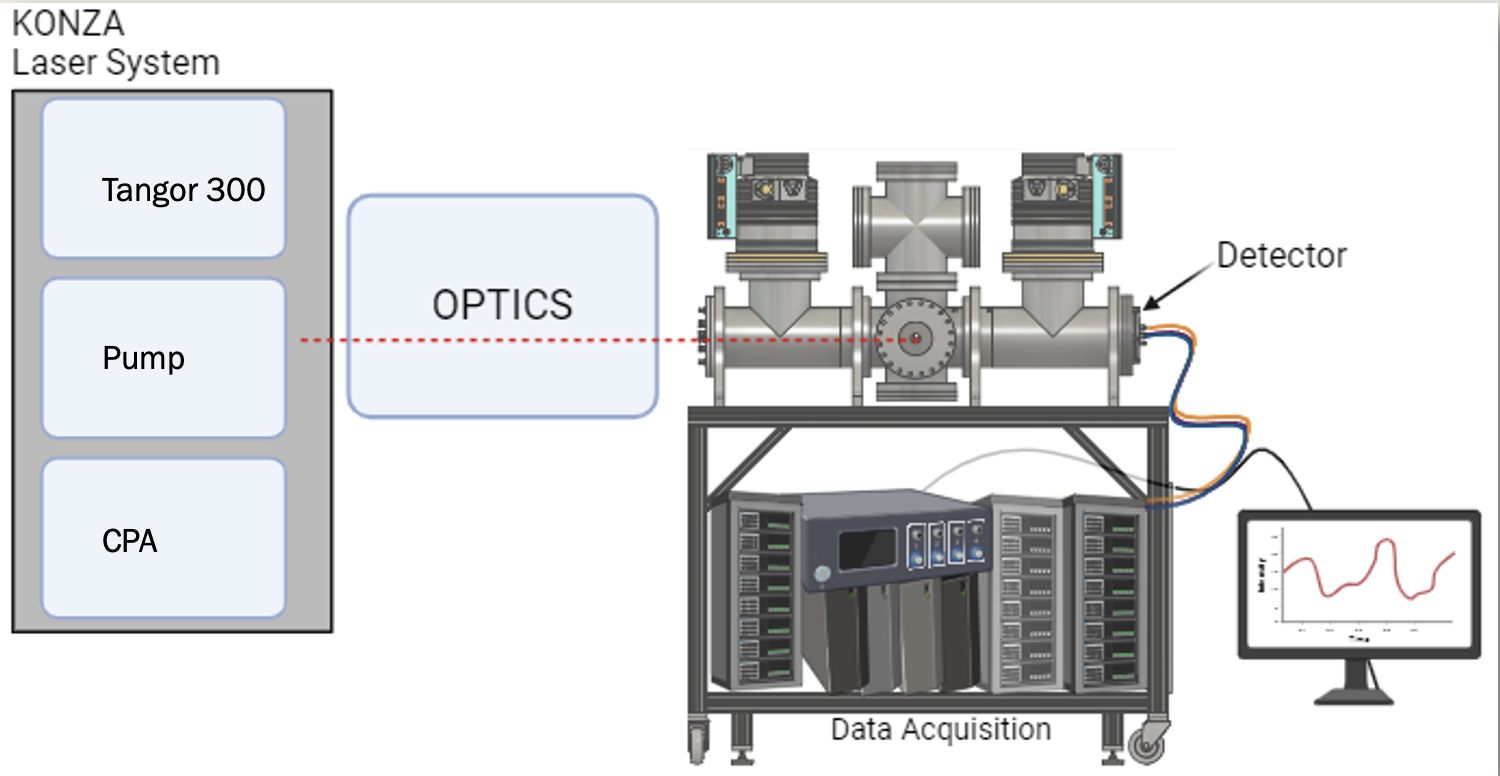
Fig. 1. Konza laser system setup, including the optics system, the D-TOF, and data acquisition system.
The laser system used to create our scans is composed of four different parts. The laser system contains the Tangor 300, the CPA, and a pump. The next step is the laser optics, which transforms the light from the laser room to the D-TOF chamber. The D-TOF is a 1-meter by 0.5-meter chamber where the spectroscopy takes place. Using laser-induced electron diffraction, the electrons or ions of a given molecule travel to a detector where the motion is converted to electrical impulses. Using a data acquisition system, we can then analyze the data on a computer. Figure 1 is a general setup of the whole system.
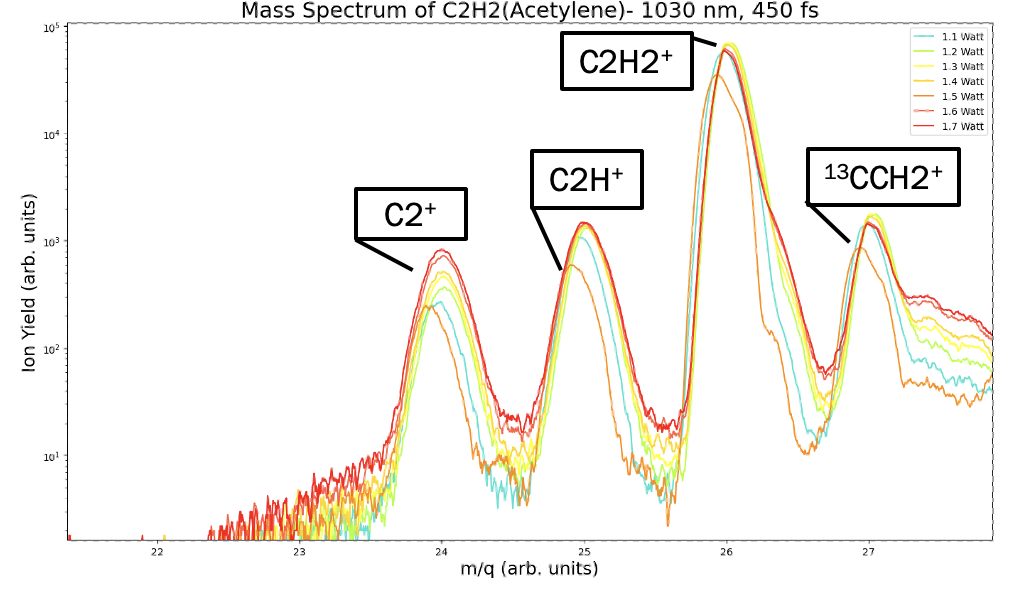
Fig. 2. Mass spectrum plot of Acetylene.
The D-TOF is a multi-use chamber where it can be used for ions or electrons. In this case, the mass spectrum setup consists of six field plates, with two of them at voltages of 240V and 450V. The voltage applied helps the heavy ions travel to the detector. Once counts are converted to the computer system, the next step is calibrating the MS data. Through the use of coding, I was able to create a script that generates a mass spectrum of given data for any hydrocarbons. Figure 2 is an example of acetylene's mass spectrum plot I created. One of the main points of MS is to verify the compounds within the chamber. Another aspect is tracking how the molecules break apart into ions, allowing us to track the relative ratios. We can conclude the patterns of breaking apart of molecules into their smaller subsections.
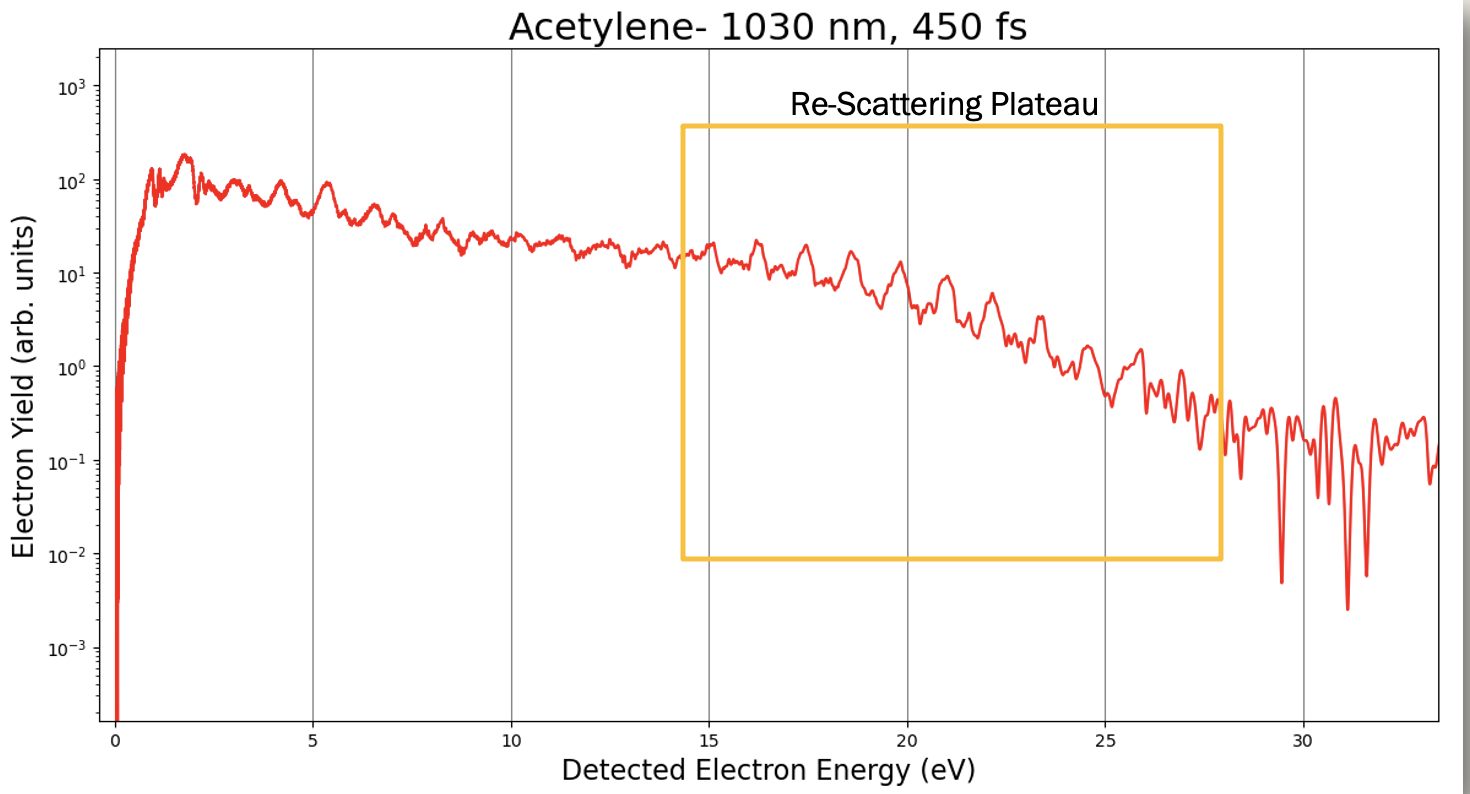
Fig. 3. Photoelectron spectrum of Acetylene, noting the re-scattering plateau.
The setup of the PES is similar to the MS setup, but without any voltages applied to the field plates. Since the laser will ionize the molecule, it will shoot off electrons with high enough kinetic energy to travel to the detector on their own. Calibration of the PES involves simple derivations of the kinetic energy equations. The biggest significance of this is observing the multiphoton ionization regime, specifically the scattering plateau, shown in Figure 3. The reason why it is so interesting for us as a group is that, classically speaking, there should not be a plateau on this plot, but almost all data shows this plateau. With that in mind, theorists are working on a reasoning for this phenomenon.
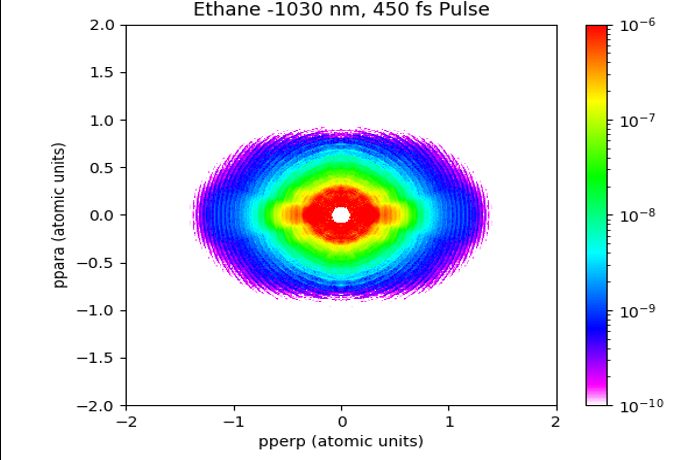
Fig. 4. Photoelectron angular distribution of ethane.
A PAD is a reconstruction of a PES. Using code and distribution angles, we can piece together a graphic. Figure 4 is what a constructed PAD looks like. The low-momentum electrons are direct electrons that have not scattered and are not useful in our analysis. Our main focus is on the outer region of the PAD where their diffraction patterns show up. Using concepts of Young's double-slit experiment, we can deduce a lot of information concerning the given molecule in question. For my three molecules tested, there is just enough information to identify which molecule is which. Further examination is still taking place.
Conclusion
In the end, I was able to look at the final plots of the MS, PES, and PAD. From these plots, our group gained a better understanding of simple hydrocarbon molecules. With the technology available, we can obtain top-of-the-line scans and plots.
Acknowledgements
I would like to thank Cosmin Blaga for allowing me to join his group. Sajed Hosseini, Pavan Muddukrishna, Eric Mullins, Frank Genty, and Koby Harding were a huge part in having such a great experience. Kim Coy, Bret Flanders, and Loren Greenman, thank you for allowing me to join for the summer. This material is based upon work supported by the National Science Foundation under Grant No. 2244539. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.