Coloumb Explosion Imaging of Ethylene with 100 kHz Laser
Nick Ihrke, Macalester College, Chemistry Major
Mentored by Dr. Artem Rudenko and Dr. Daniel Rolles
Coulomb explosion imaging (CEI) is a molecular imaging technique that is useful for studying the structure of molecules as well as the mechanisms of some chemical reactions. CEI relies on using a strong laser field to completely or near completely ionize target molecules. The resulting fragment ions will coloumbically repel one another and 'explode' outward. Specialized detectors acquire information about the time-of-flight (TOF) and final position of ion fragments. Using this information, as well as the conservation of momentum, we can calculate the classical kinetic energies and momenta of our ion fragments. Newton plots allow us to visualize specific breakup channels and provide us an image of the molecule as it explodes. Now, following the acquisition of the new 100 kHz KONZA laser, CEI experiments can be performed in a fraction of the time they used to take. Moreover, it is possible that with higher count rate data, more complicated structures can be analyzed.
In order to produce CEIs we make use of a cold target recoil ion momentum spectroscopy (COLTRIMS) setup. We send a low pressure jet of molecules with heavily restricted degrees of freedom (hence cold) into the path of a femtosecond laser. The laser shot ionizes the target molecule and the positively charged fragments are directed towards a detector by a powerful electric field. A microchannel plate (MCP) detector collects and amplifies the signal of an ion hit. This signal is referenced against the initial laser pulse to give an ion TOF value. A delay line which consists of two coils of wire wrapped perpendicular to one another allows us to measure the position of an ion hit by measuring the time it takes for the signal to reach each corner of the detector.
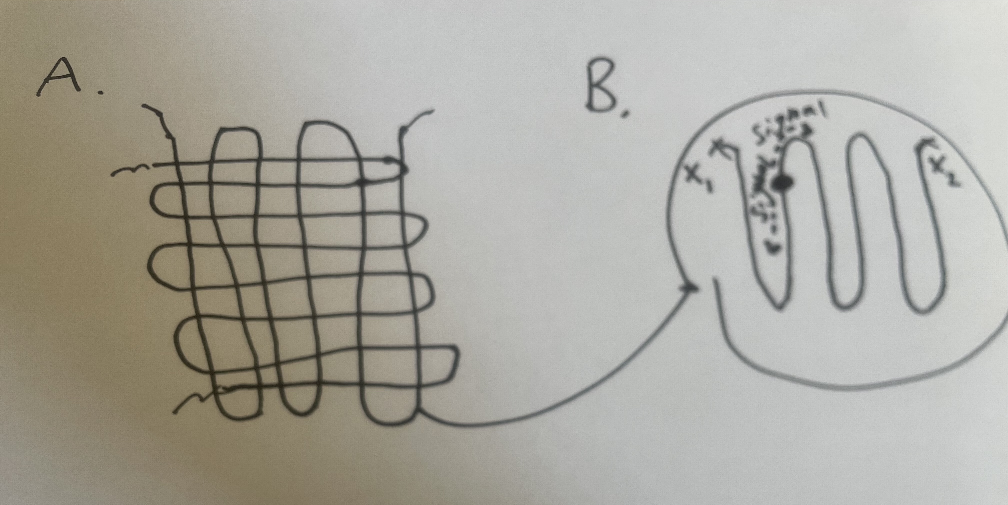
Fig. 1. A. Cartoon of what the wires in a delay line look. B. Signal propogating through one wire in a delay line. Note that each point along the wire has a characteristic time for each signal to reach each corner labeled x1 and x2.
These TOF values vary depending on the mass and charge of the incident ion fragments. We can plot the number of ion counts against the TOF producing a mass spectra. These spectra are useful for identifying what fragments are being produced. It is also important to see how different experimental conditions affect the populations of different ions.
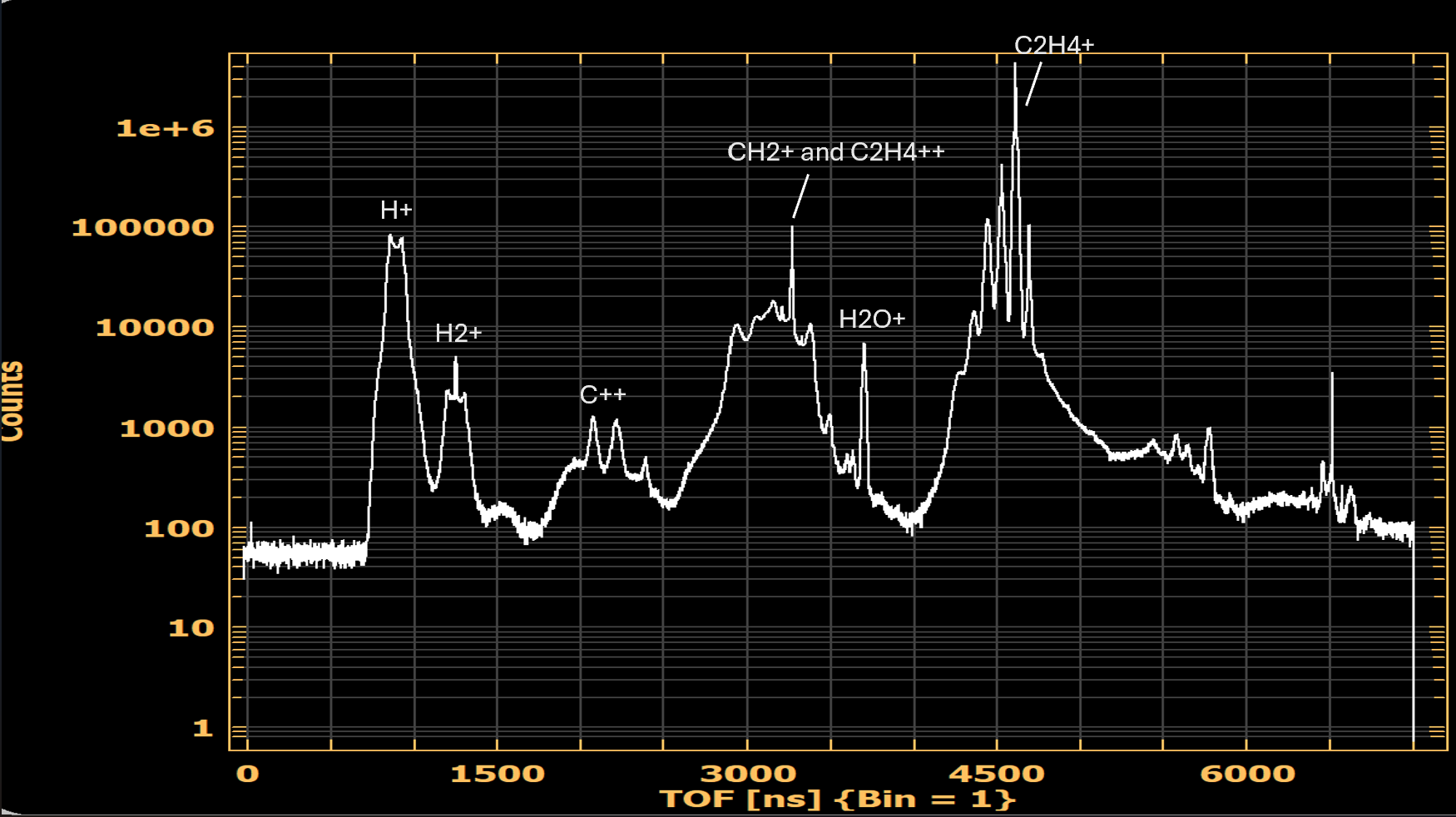
Fig. 2. TOF is proportional to the mass over charge ratio of incoming ions. Each peak in this spectra corresponds to a different ion produced by interaction with a laser pulse. Key ion peaks are labeled here.
We can also plot a 2D distribution of TOF’s against the X and Y position of the ion hit. These plots produce ring shaped structures indicative of the 3D spherical distribution of ions in momentum space. For instance, in a two body breakup channel one ion will travel ‘towards’ the detector as the parent molecule explodes. The other ion will travel in exactly the opposite direction ‘away’ from the detector. The first ion will have a shorter TOF and the second will have a longer TOF.
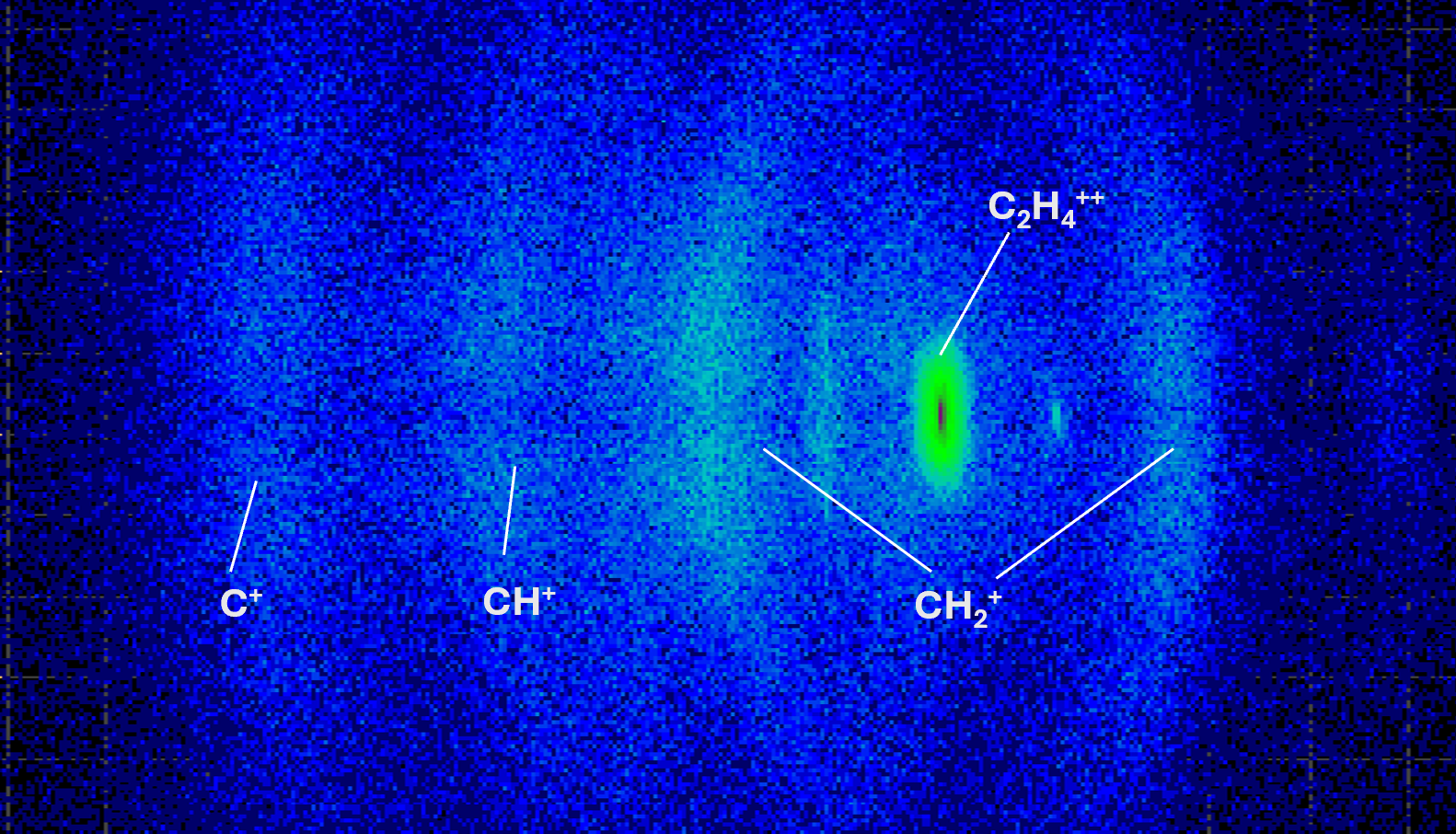
Fig. 3. Each ring corresponds to a different ion’s TOF. Key ions for ethylene are labeled in the figure.
Each ring corresponds to a different ion’s TOF. Key ions for ethylene are labeled in the figure.
By plotting the time of flight of coincident ions against one another we get photoion photoion coincidence (PIPICO) spectra. Sharp diagonal lines indicate that a channel is complete (that is, contains all atoms of the original molecule) and that the channel follows the conservation of momentum. Thus, by analyzing these lines we can ensure that our data reflects ion pairs from the same molecule. Blurrier lines suggest breakup into three or more bodies.
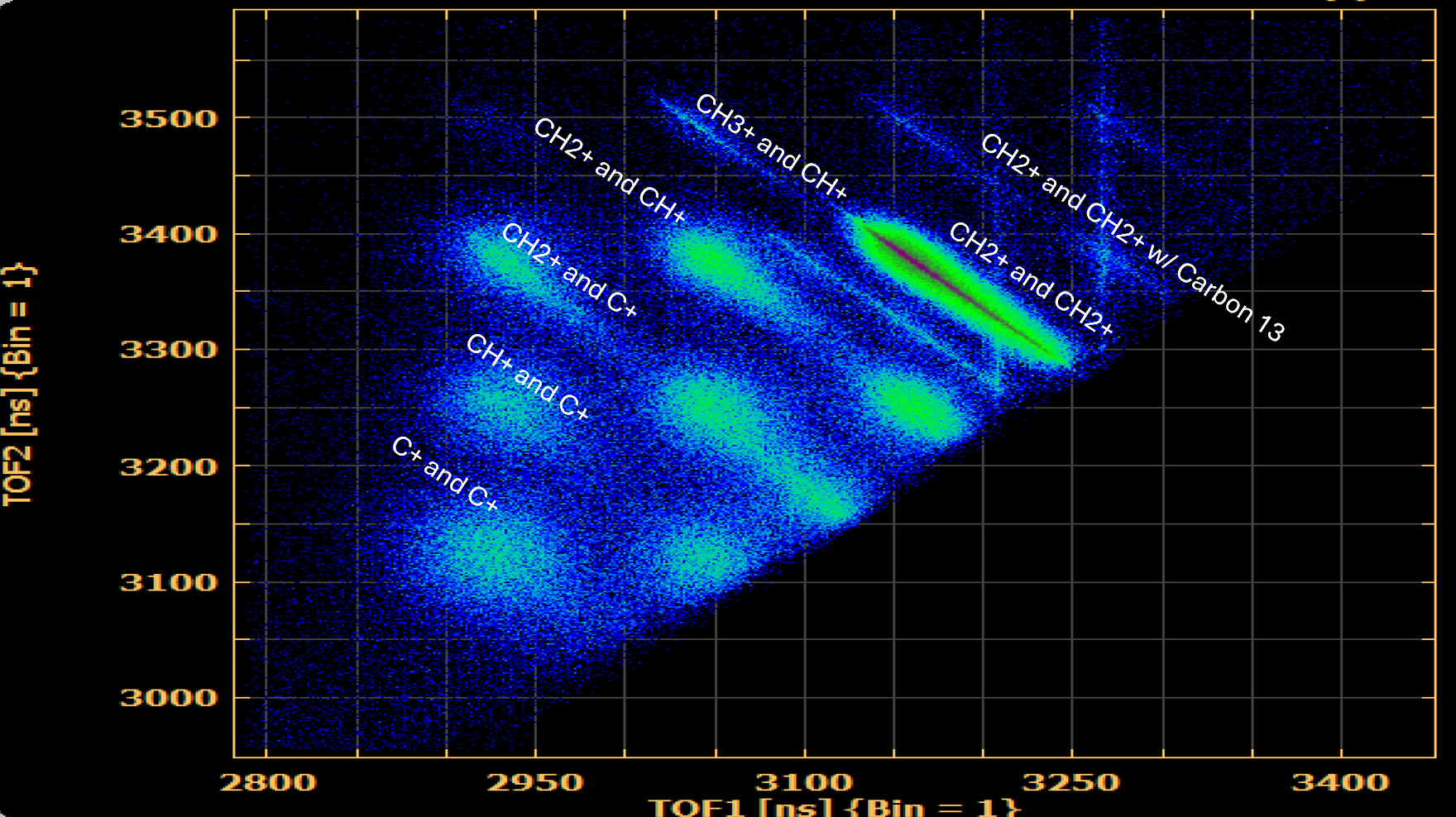
Fig. 4. Key PIPICO lines for ethylene are shown here.
A key figure of merit for CEI is the kinetic energy released or KER of the explosion. In a classical picture this energy should be equal to the potential energy stored between the two charged ions. Here, this potential energy is transferred to the kinetic energy as the ions repulse one another. Smaller ions will move faster as a result of Coulomb explosion and consequently have a larger contribution to the overall KER.
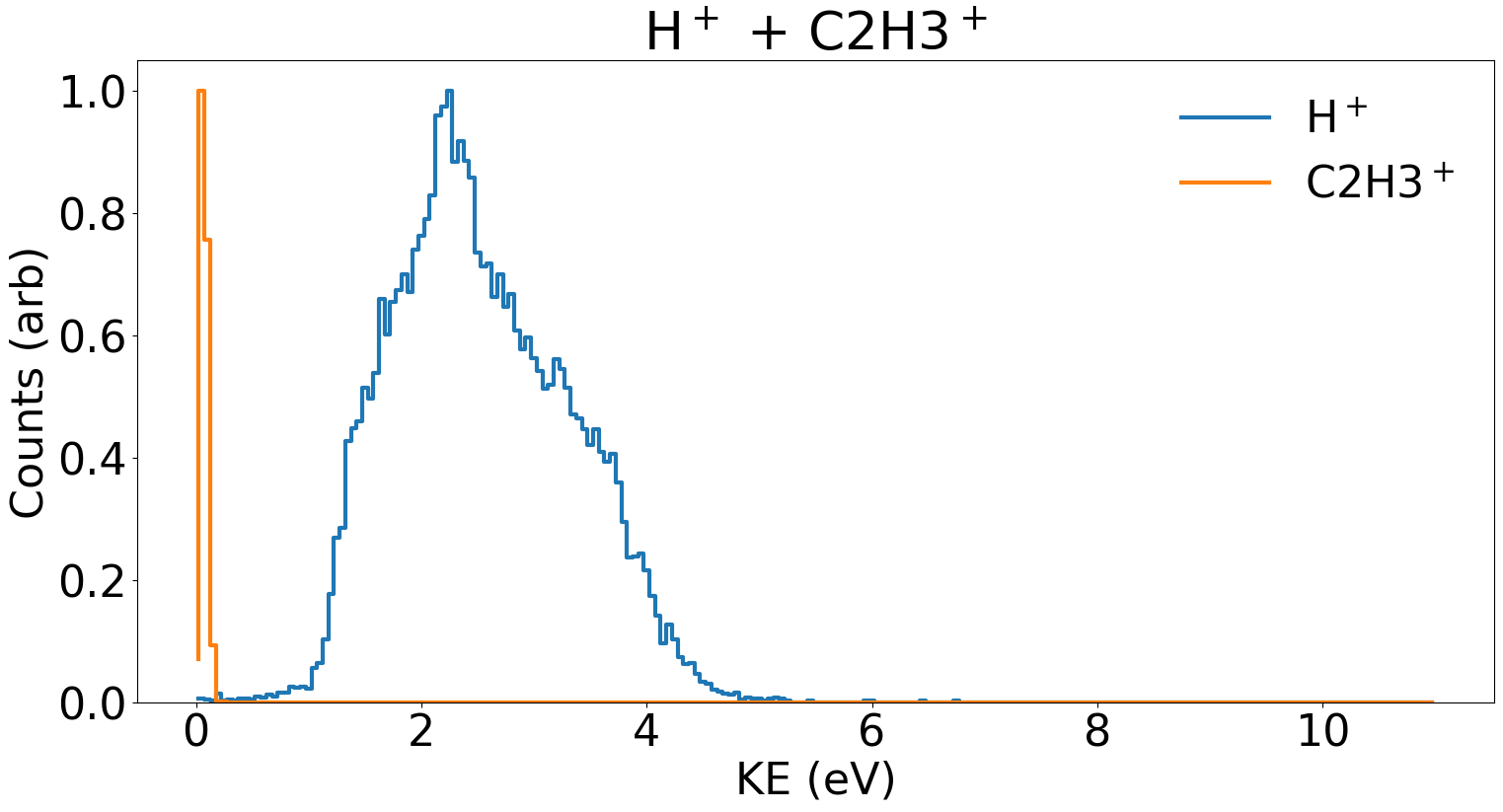
Fig. 5. A KER plot for the H+ and C2H3+ fragmentation channel in ethylene. Note that H+ contributes a larger portion of the overall as compared to C2H3+.
Finally, Newton Plots show the momenta of ions created during Coulomb explosion. For high symmetry chemical systems, where particles fly away from each other in a predictable way, the Newton Plot creates a ball and stick picture of the original molecule. In more complicated structures however it is harder to intuit what a CEI will look like.
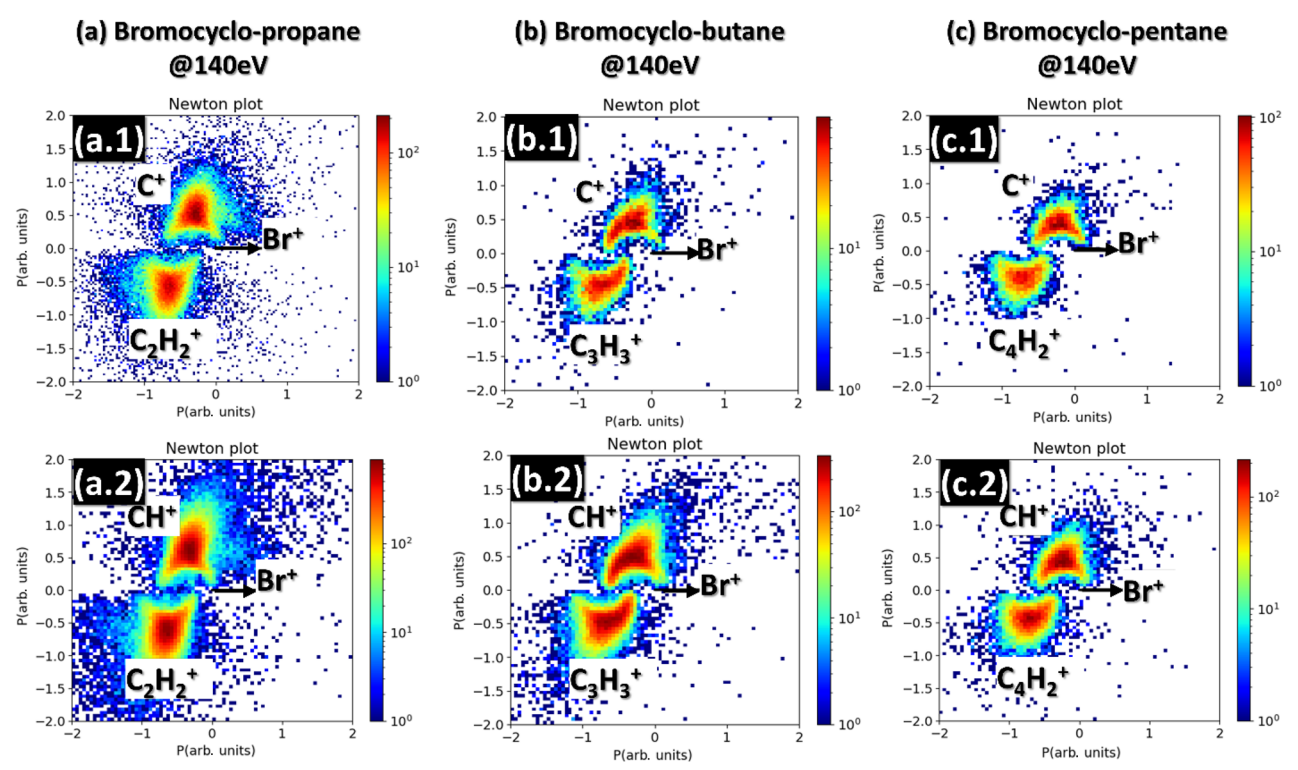
Fig. 6. Examples of Newton plots for halogenated alkane rings from "Hydrogen migration in inner‑shell ionized halogenated cyclic hydrocarbons" by Rolles et al. published in Scientific Reports in 2023.
References
[1] R. E. Goetz, C. P. Koch, and L. Greenman, Phys. Rev. Lett. 122, 013204 (2019).
[2] F. A. Gianturco, R. R. Lucchese, and N. Sanna, J. Chem. Phys. 100, 6464 (1994).
[3] A. P. P. Natalense and R. R. Lucchese, J. Chem. Phys. 111, 5344 (1999).
[4] R. E. Goetz, C. P. Koch, and L. Greenman, arXiv: 2104.07522 (2021).
Acknowledgements
I am very grateful for the mentorship and guidance provided by both Artem Rudenko and Daniel Rolles. Thank you also to Sanduni Kudagama, Smita Ganguly, and Tu Nguyen for letting me learn from them in the lab and answering my questions. Finally, thank you to Loren Greenman, Bret Flanders and Kim Coy for organizing this REU program. This material is based upon work supported by the National Science Foundation under Grant No. 2244539. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.