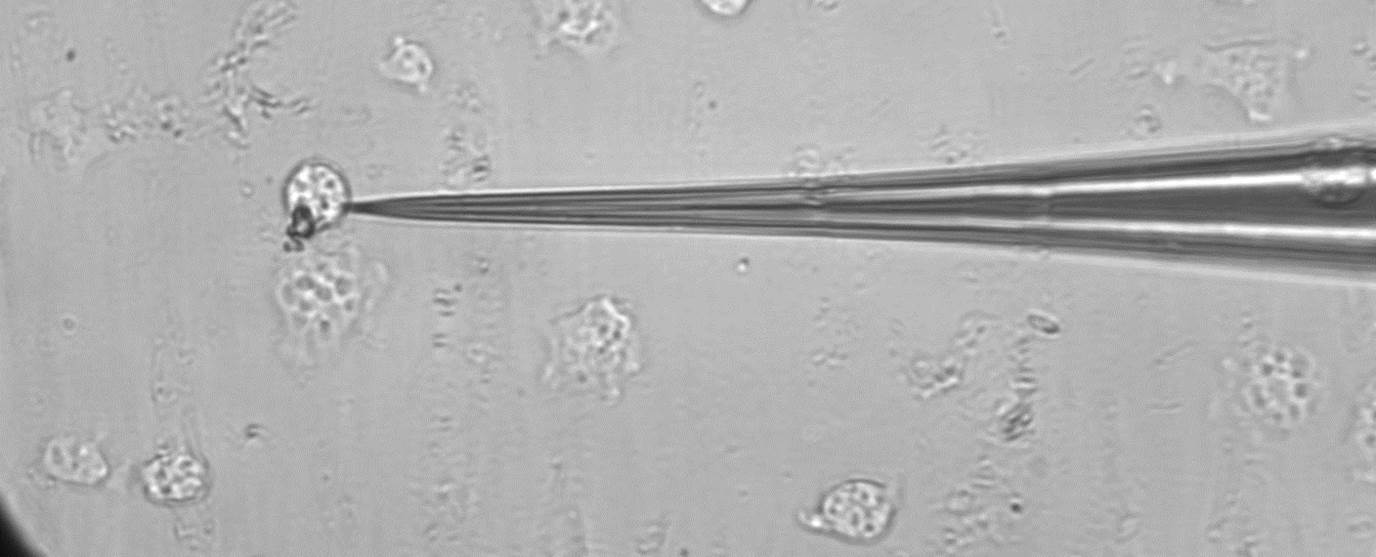
Interfacial Tension of Amoeba Cells Settling
By Angelynn Simenson
Supervisor: Bret Flanders, Associate Professor of Physics
Kansas State University Physics Department REU Program

This program is funded by the National Science Foundation through grant number PHYS-1461251. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Project Overview: The
purpose of determining the interfacial tension of amoeba cell settling is to
understand cell migration via cell adhesion to further elements of the medical
field such as cancer treatments by studying the breakup of cancer cells. To
reach this broad goal, we first need to take smaller steps to get there. It is
important to understand the process of cell settling before determining the
interfacial tension of the amoeba cell. A cell begins falling toward a glass
surface due to the force of gravity. When the cell comes into contact with the
glass, two different forces act on it. The uncompensated young force, which is
due to the interactions of the molecules, acts outwardly on the spreading cell.
The viscous force acts inward on the cell. When these two forces are equal, the
cell has settled, and forms an equilibrium contact angle with the substrate.
There is a time,![]() , that determines how long it will
take a settling cell to reach this equilibrium contact angle. This time is
called the rise time. The rise time can be written as a function of constants,
of which all are known except the equilibrium contact angle and the interfacial
tension. The interfacial tension is what will be determined by this project.
Below is a diagram of cell settling, and a graph of
, that determines how long it will
take a settling cell to reach this equilibrium contact angle. This time is
called the rise time. The rise time can be written as a function of constants,
of which all are known except the equilibrium contact angle and the interfacial
tension. The interfacial tension is what will be determined by this project.
Below is a diagram of cell settling, and a graph of ![]() , that illustrates how rise time occurs.
, that illustrates how rise time occurs.
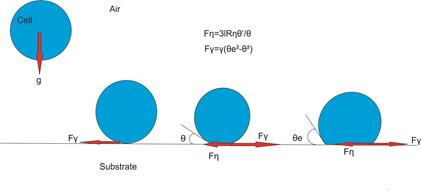
Cell Settling
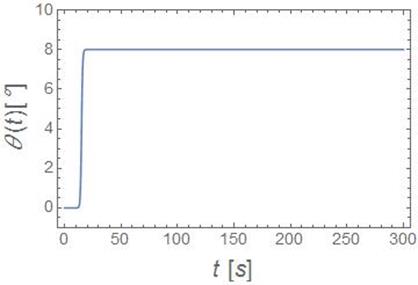
Graph of ![]()
Research Description: At K-State I am working on determining the interfacial energy tension of amoeba cells. This is a constant that arises from the molecular interactions between materials because of the energy stored in the bonds in the material and between materials. To determine this constant and test the purposed theory, I designed a microfluidics chamber that we will use. The chamber will have a spot for the fluid containing the cells and a 1mm square channel for the fluid to flow into a reservoir. Attached to the reservoir will be a pump that will create a changeable fluid velocity. This will allow us to use the forces to determine the interfacial tension. This is done by equating the critical force on a tube of pulled cell membrane with the friction force to determine the interfacial tension by determining the velocities when the forces equal out.
Research Progress: I have made good progress on this project; though have run into some bumps in the road, as happens with any project. Over the weeks of the program, my focus has shifted from understanding theory to designing the experiment.
In the first week I was focused on understanding the forces that act on a settling cell by deriving the uncompensated Young force and the viscous force acting on the system.
I spent the second week equating these two forces, setting up, and solving
the differential equation for the contact angle, ![]() . It is here that the rise time can be
determined and it is seen that the only two parameters are
. It is here that the rise time can be
determined and it is seen that the only two parameters are ![]() .
.
In the third week, I spent time researching procedures to test the interfacial tension, and understood that using an acrylic chamber to extrude a tube of membrane would be the most feasible setup. I also began designing the bottom piece of the apparatus this week.
I submitted the design to the machine shop in the fourth week, and ordered the glass rods that we needed to make microrods and the silicone kit needed to make the top piece. The bottom piece of the instrument is pictured below.

Fluid flow chamber
In the fifth and sixth weeks, I worked on developing a process for making the silicone sheet for the top piece of the apparatus, and worked more on the presentation of this project. During this time we had to wait for the PDMS to arrive. A chunk of the PDMS is pictured below.

PDMS cover
In the seventh week of the program, I perfected the process for making the silicone top by finding the appropriate curing time and ratio of PDMS to curing agent. We modified the design of the bottom piece of the apparatus so that we could obtain a closer working distance with our microscope lens. Later in the week, we pulled the glass microrods needed to pick up a cell from which the membrane tether will be pulled. I also ordered the lectin, a protein that will be used to adhere the cell to the microrod. To pull the glass microrods we used the machine pictured below that uses a hot filament to melt the rod and weights to pull the tips from the molten glass.

Microrod puller
In the eighth week, I established a process for operating the pump that will create our changing fluid flow. I also ordered tubing to hook the pump up to the instrument. We got the entire instrument set up and were just waiting on the lectin. Pictured below is the complete setup of the experiment on the microscope.
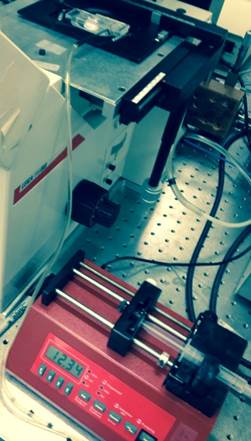
Flow chamber connected to pump
We successfully pulled a tether and altered our process for picking up cells in the ninth week. We pulled more microtubes, rather than microrods, and developed a suction approach, also using a syringe, to adhere the cells to the microtube. We decided to use a 60 mL syringe rather than a 5 mL to increase the time we could run the experiment, so that we did not have to stop mid tether. Pictured below is the suction system that was created.

Syringe pump creating suction
In the last week, we used the methods from Europhys. Lett., 64 (6), pp.
837–843 (2003), Hydrodynamic Extrusion of Tubes from Giant Vesicles, assuming a
value for the bending modulus, ![]() , to find the interfacial tension. This
was a bit high, so we used a parameter
, to find the interfacial tension. This
was a bit high, so we used a parameter ![]() , the distance between the contact point
and the intercept of a line fitted to the cell based on its shape, and
substituted that in for
, the distance between the contact point
and the intercept of a line fitted to the cell based on its shape, and
substituted that in for ![]() , and then solved using similar methods
to the paper. This gave us a more expected value of
, and then solved using similar methods
to the paper. This gave us a more expected value of ![]() .
.
Final Presentation: This presentation covers more in depth what I have stated on the webpage. The PowerPoint can be found here
Lectures: There have been a variety of interesting lectures about the various subfields of physics. Some of my favorites have been:
Chris Sorenson, in condensed matter, gave a lecture on nanoparticles. He made it very easy to understand by relating the color of nanoparticles to everyday things, like the color of the sky and the clouds in it. He explained that the particles in the cloud are large and use the particle nature of light, and thus appear white, while the particles in the gases in the sky are smaller and reflect blue. If the particle is smaller than the wavelength, the wave nature of light must be used to analyze the particles.
Glenn Horton-Smith, in high energy physics, gave a lecture on neutrinos, which was at times very complicated, but extremely interesting. He discussed how the neutrino is responsible for the conservation of energy in nuclear reactions, giving some of its properties, and how the small components of particles, such as down quarks, can produce electron neutrinos. To conserve energy, some of the properties are neutrinos must have no charge, mass, or nuclear interactions, but must carry momentum and energy and be a fermion.
Tim Bolton, also in high energy physics, gave a very interesting lecture on why we must have the Higgs boson. He started out by explaining some of the simpler principles in quantum field theory, progressing to gauge theory, which explains charge and current conservation. It is gauge invariance that makes E&M renormalizable, but as it nturns out gauge invariance only works without mass. The Higgs provides a solution to this problem, as forces are dependent on mass and acceleration.
Jeremy Schmit, also a biophysicist, gave a lecture on protein folding models using computational approaches. While this lecture was easy for me to understand as a prospective biophysicist, Dr. Schmit also made it easy for the students from other subfields to understand by discussing why proteins fold, why the research is important and a simplified model of a folding protein. The most interesting thing to me was his discussion of Alzheimer’s disease and what proteins play a role in that, the theory of their function, and the reason for such a slow onset.
There was one lecture I had quite a bit of difficulty understanding, and that was Bharat Ratra’s lecture on cosmology and the expansion of the universe.
Ethics Class: There have been some interesting discussions in the ethics class that have sparked a fair amount of debate. Among them were the discussion of the lack of diversity in physics, why it is there, and how to improve it. A second topic that sparked a long discussion was how to determine authors on a paper and correct citations of one’s own work. Thirdly, the discussion of the mentor-mentee relationship, and whether it should strictly be professional, and who determines that sparked a heated debate.
About Me: I am a Physics major at the University of Missouri. I will be entering my senior year in the fall of 2015. My primary research interests are in membrane and neuro biophysics. I decided to participate in the K-State REU to gain new knowledge of experimental techniques in these fields. I plan to pursue graduate studies in one of these two areas.
I chose to pursue physics due to its ability to explain the inner workings of everyday things that we take for granted, like televisions, cellphones, and medications.
I chose to study biophysics after realizing that there are a lot of molecular interactions and properties that went into biology that could be determined with physics. Another reason that drove me to pursue biophysics was that it felt to me like I was helping improve a less trivial part of someone’s life in biophysics. I also worked in an electrical engineering lab on a transistor based vapor sensor project before working in the membrane biophysics lab that I work in at Missouri, and learned that I did not like it.

I have found the following links particularly informative or useful:
American Physical Society Statements on Ethics