Physicists publish research on ultrafast dynamics in a molecular photoswitch in Nature Chemistry
An international collaboration led by researchers from the K-State Physics Department and the Oxford University Chemistry Department has studied ultrafast processes in the molecular photoswitch quadricyclane, published in Nature Chemistry.
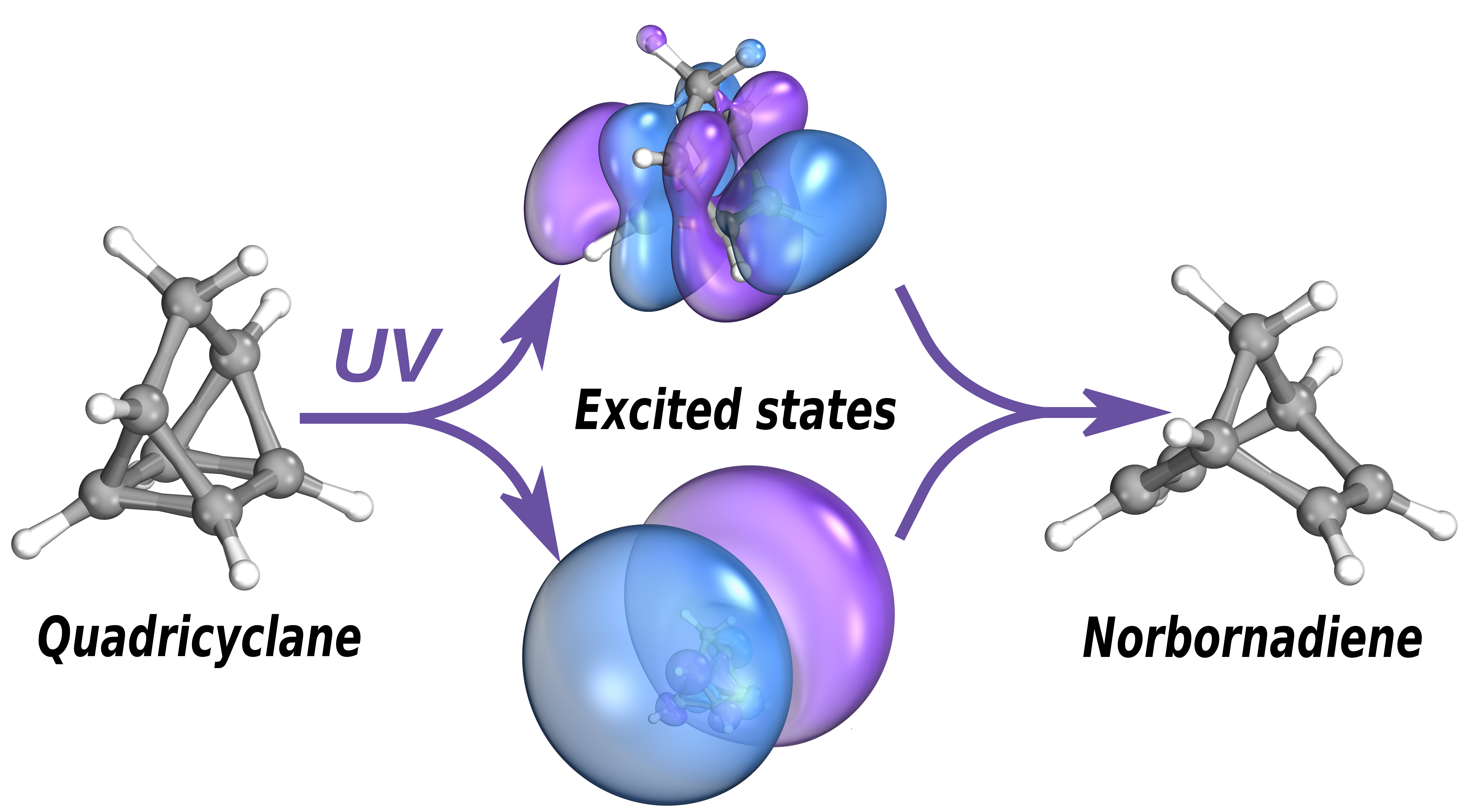
Quadricyclane and norbornadiene are two isomers, that is molecules with the same chemical formula but different geometry, which interconvert upon absorption of ultraviolet light. Quadricyclane is the higher-energy isomer and can be used to store solar energy, which is then released by a catalytic process as heat. So-called molecular solar thermal (MOST) devices exploit such isomer pairs to, for instance, warm houses by absorbing energy from the sun during the day and then releasing it at night.
The experiment, led by K-State physics professor Daniel Rolles and his graduate student Kurtis Borne, used ultrashort light pulses produced by the FERMI free-electron laser facility in Trieste, Italy, to track the conversion between the two isomers on a timescale of femtoseconds. The experimental results were interpreted with the help of state-of-the-art computer simulations done in Oxford. The combined study discovered faster than previously identified pathways leading to the conversion, which may help designing new ways of controlling the outcome and efficiency of this important class of photoreactions.
Read the full paper in Nature Chemistry.