The Mathematical Modeling of the Photoionization of Dichloromethane
Stephen Tivenan, University of Mary Washington, Physics and Mathematics Major
Mentored by Dr. Loren Greenman
By calculating the optimal energy values for two possible photoionization channels of dichloromethane (CH2Cl2), we attempted to show that, between the two channels, there exists a more probable channel. The two tested channels were the ion of methylene (CH2 +) with chlorine (Cl2) and methylene (CH2) with the ion of chlorine (Cl2 +). In order to find the favored path, we constructed potential energy curves for each of the individual pieces of the channel, and the ion of dichloromethane. In comparing the total sum of the optimal value of the energies for each channel to the energy of the dichloromethane ion, when internuclear distance is far apart, we can approximate the composition of the dichloromethane ion when broken apart. The calculations that were produced used the Born-Oppenheimer and the Hartree-Fock Approximations. The carbon and the two chloride internuclear bond distance varied between 1-5 Angstroms, while also varying the symmetry of our wavefunction. Thus, we produced multiple excited states for dichloromethane and produced one excited state for dichloromethane ion. Our calculations suggest that the ground state of the dichloromethane ion may favor the channel which contains the chlorine ion and the methylene molecule.
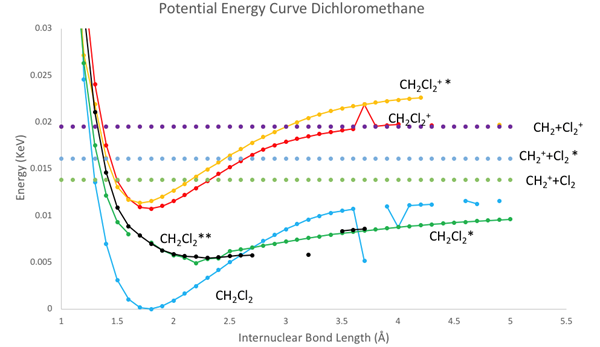
The figure above shows the many potential energy curves of dichloromethane and the dichloromethane ion that were produced in the experiment. The horizontal dots are the sum of the energy of the fragments. The asterisks by the name of each potential energy curve and the name of the horizontal energy dots denotes the molecules at a different symmetries. The y axis energy values are based upon the lowest energy value of the ground state of dichloromethane, while the x axis is the internuclear distance between the carbon chloride bonds. Notice that as the internuclear distance increases to infinity, the purple line seems to converge to the energy value of the dichloromethane ion. Thus, this indicates that CH2 + Cl2 + would be the fragments of the dichloromethane ion.
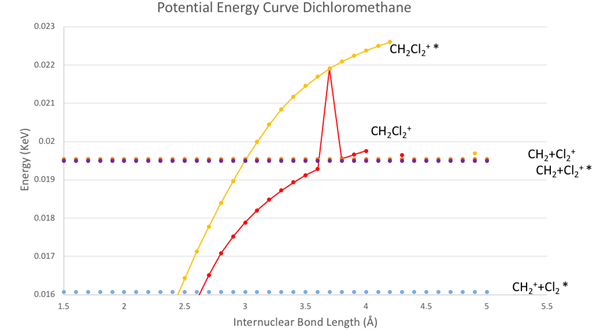
Looking at the potential energy curve even closer, we see that our potential curve is incomplete and jagged. In order to improve the curves, further research could use other approximations other than the Hartree-Fock Approximation. Also, there are two energy values of different symmetries which the dichloromethane ion may converge to. Thus moving forward, we can produce more energy values at different symmetries of the methylene, chlorine, and their ions to generate more possible converging energy values to our potential energy curves.
Acknowledgments
Many thanks to Dr. Greenman or guiding me through this project, the entire team of the Kansas State Physics REU Program, and the National Science Foundation. I learned a great deal during my participation in this program and was inspired by both the excellent teaching and very interesting and varied research projects.