Quantum Control of Photoelectron Circular Dichroism on Limonene
Julian A. Suarez Cardona, University of Nebraska-Lincoln, Physics Major
Mentored by Dr. Loren Greenman
The purpose of my research is focused on using quantum theory and computational tools to better understand the behavior of the chiral molecule Limonene, following the paper 'Quantum Control of Photoelectron Circular Dichroism' [1]. The main objective of recreating the methods of this paper on Limonene was to observe the results on a larger and more complex molecule, in order to compare them to the results found when applied to CHBrClF.
My research is based on a theoretical case study for proving chiral molecules with circularly polarized light. A randomly orientated molecule (CHBrClF in Figure 1) in the ground state is ionized by a circularly polarized, left-hand polarization denoted as positive (+) and right-handed polarization as negative (-), emitting an electron that is measured on a detector plane forming a photon-electron angular distribution. We then take the difference from the left-circularized and right-circularized photo-electron angular distribution to obtain the PECD.
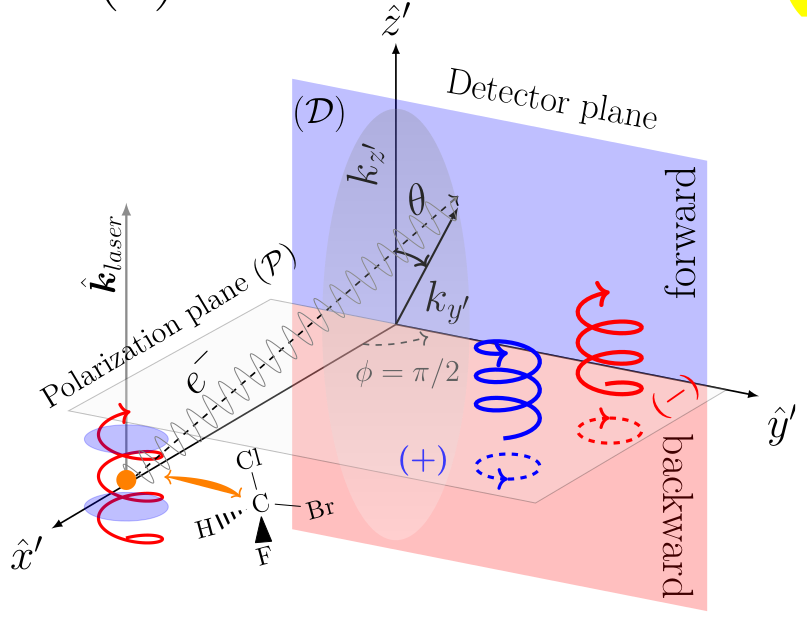
Figure 1: Photoionization setup
The research is divided into two main components, time-independent description of the molecule and the interaction of the molecule with a time-dependent laser pulse. The time-independent description is comprised of two stages, computing for the molecule energy eigenvalues (molecular orbitals) and the photoionization. For the energy eigenvalues I made use of the Hartree-Fock approximation, which can be simplified as an approximation used to make a multi-electron system into a single electron system by approximating the coulomb interaction between all electrons to the interaction of one electron with the average value of all electrons. This procedure was accomplished using the code depicted below on MOLPRO, with the aug-cc-pVDZ basis set.
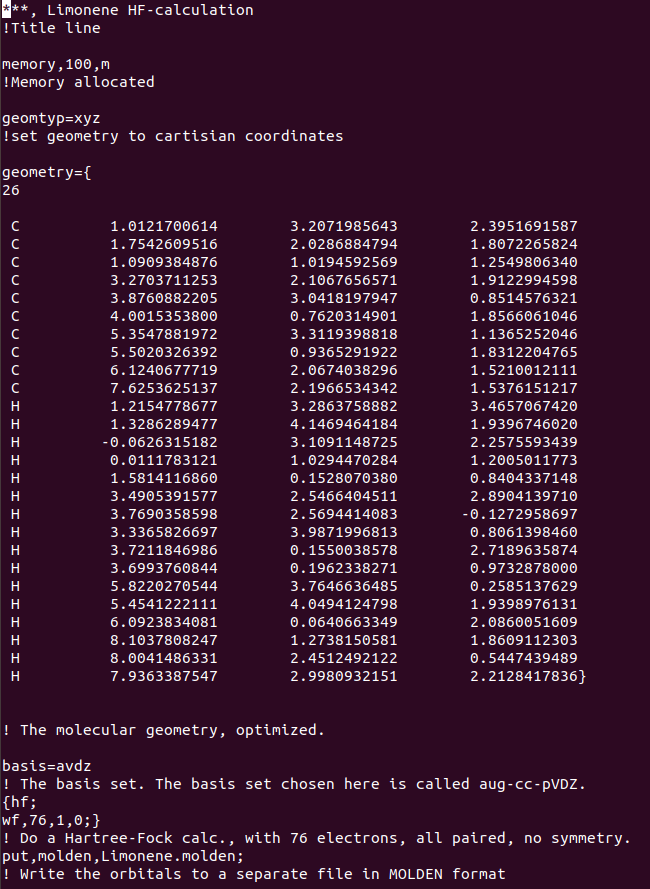
Figure 2: Molpro Code
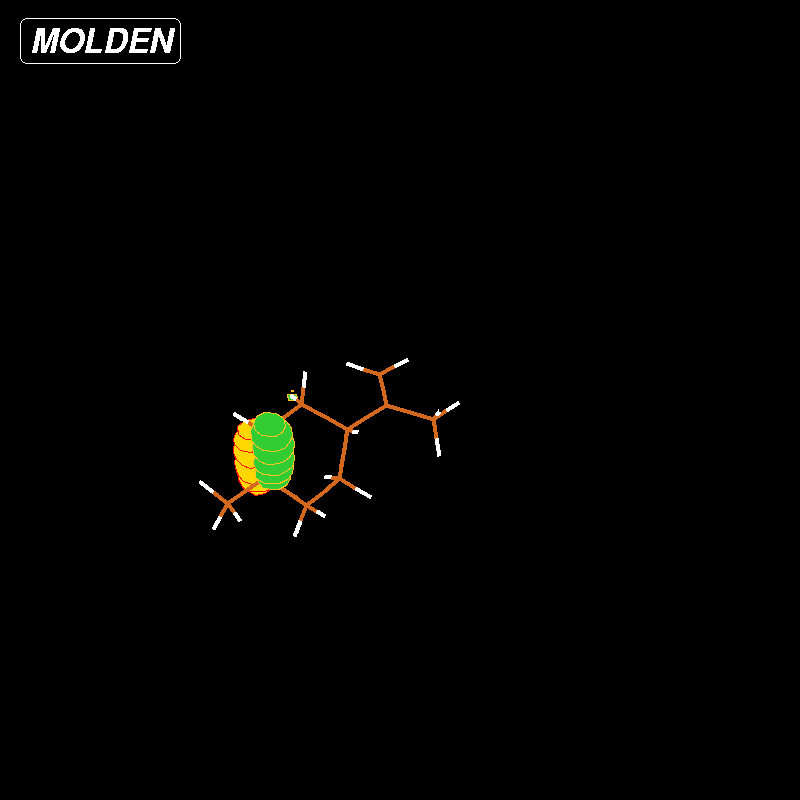
Figure 3: HOMO Limonene
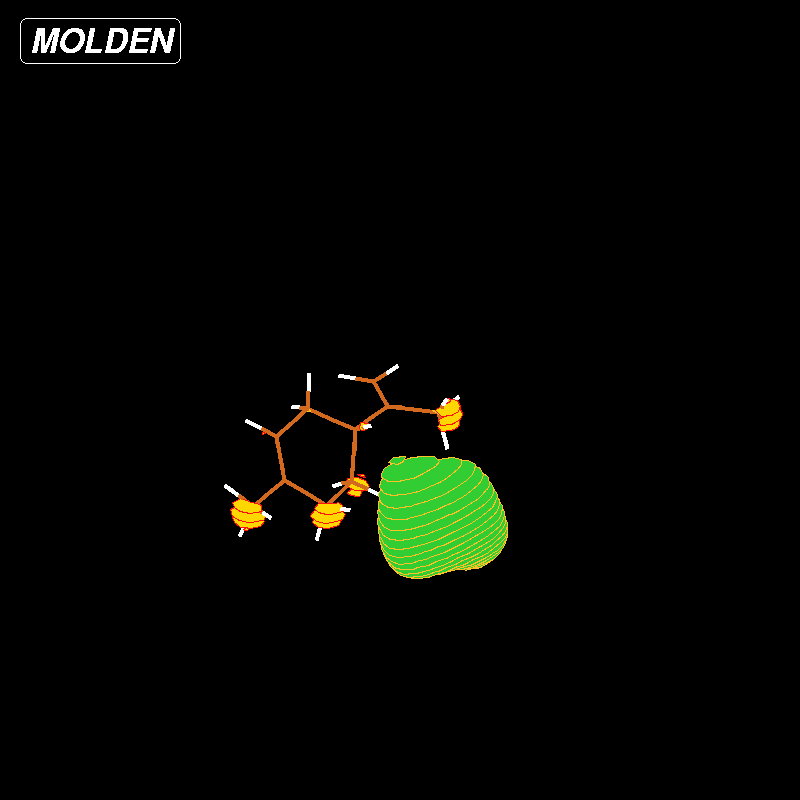
Figure 4: LUMO Liminene
This matrix gives us information of the electric dipole moment generated when the molecule is ionized. The resulting matrix is valuable because the direction of the electric dipole moment gives the polarization of the transition, which determines how the molecule interacts with and electromagnetic wave with a given polarization. The next step on the research will be to begin the analysis of the interaction of the molecule with a time-dependent electromagnetic wave within the spectral bandwidth and compare the results with CHBrClF.
The photoionization of the molecule is simulated with the help of the software ePolyScat, a software used to study electron-molecule scattering. This procedure yields the photoionization dipole matrix using the code depicted in Fig. 3.
Figure 5: ePolyScat code
The next step on the research will be to begin the analysis of the interaction of the molecule with a time-dependent electromagnetic wave within the spectral bandwidth, and compare the results whit CHBrClF.
References
- Goetz, E. R., Koch, C. P., & Greenman, L. (2019, January 10). Quantum Control of Photoelectron Circular Dichroism. Physical Review Letter, 122. doi:10.1103/PhysRevLett.122.013204
- Beaulie, S., Comby, A., Descamps, D., Petit, S., Legarde, F., Fabre, B., .Mairesse, Y. (01 october 2018). Multiphoton photoelectron circular dichroism of limonene with independent polarization state controlof the bound-bound and bound-continuum transitions. The Journal of CHemical Physics. doi:10.1063/1.5042533
- Szabo, A., & Ostlund, N. S. (1982). The Hartree-Fock Aproximaton. In Modern Quantum Chemistry; Introduction to Advanced Electronic Structure Theory (p. 108149). Mineola, New York: Dover Publication. Limonene. (n.d.). Retrieved from https://webbook.nist.gov/cgi/cbook.cgi?ID=C138863&Mask=20 Libretexts. (2019, June 05). Circular Dichroism. Retrieved from https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Electronic_Spectroscopy/Circular_Dichroism Powerpoint
Acknowledgments
Dr. Loren Greenman; Dr. Esteban Goetz; Dr. Siddhartha Chattopadhyay; Dr. Bret Flanders; NERSC; Kansas State University; National Science Foundation