Temperature Dependent Solubility of Thioglycerol-Ligated ZnS Nanoparticles in 4:1 MeOH:H2O Solution
Daniel Scott
University of Houston
Physics and Mathematics Major
Mentored by Dr. Chris Sorensen
In recent decades, modern advancements in science, technology, and medicine have pushed towards ever miniaturizing devices and materials. Of significant interest is further research into various nanomaterials, that is, particles and structures ranging from single to several hundred nanometers (10,000 times smaller than an average human cell). Due to the number and complexity of interactions at this scale, computer simulations into the behavior and properties of nanomaterials is often prohibitively difficult. For this reason, direct experimentation is necessary to determine the nature of different nanomaterials and broaden our fundamental knowledge of nanosystems. Our project aims at identifying one such feature: the solubility in methanol+water of zinc sulfide (ZnS) nanoparticles (NPs) ligated (capped, for stability) with thioglycerol.
The procedure is outlined in Fig. 1.
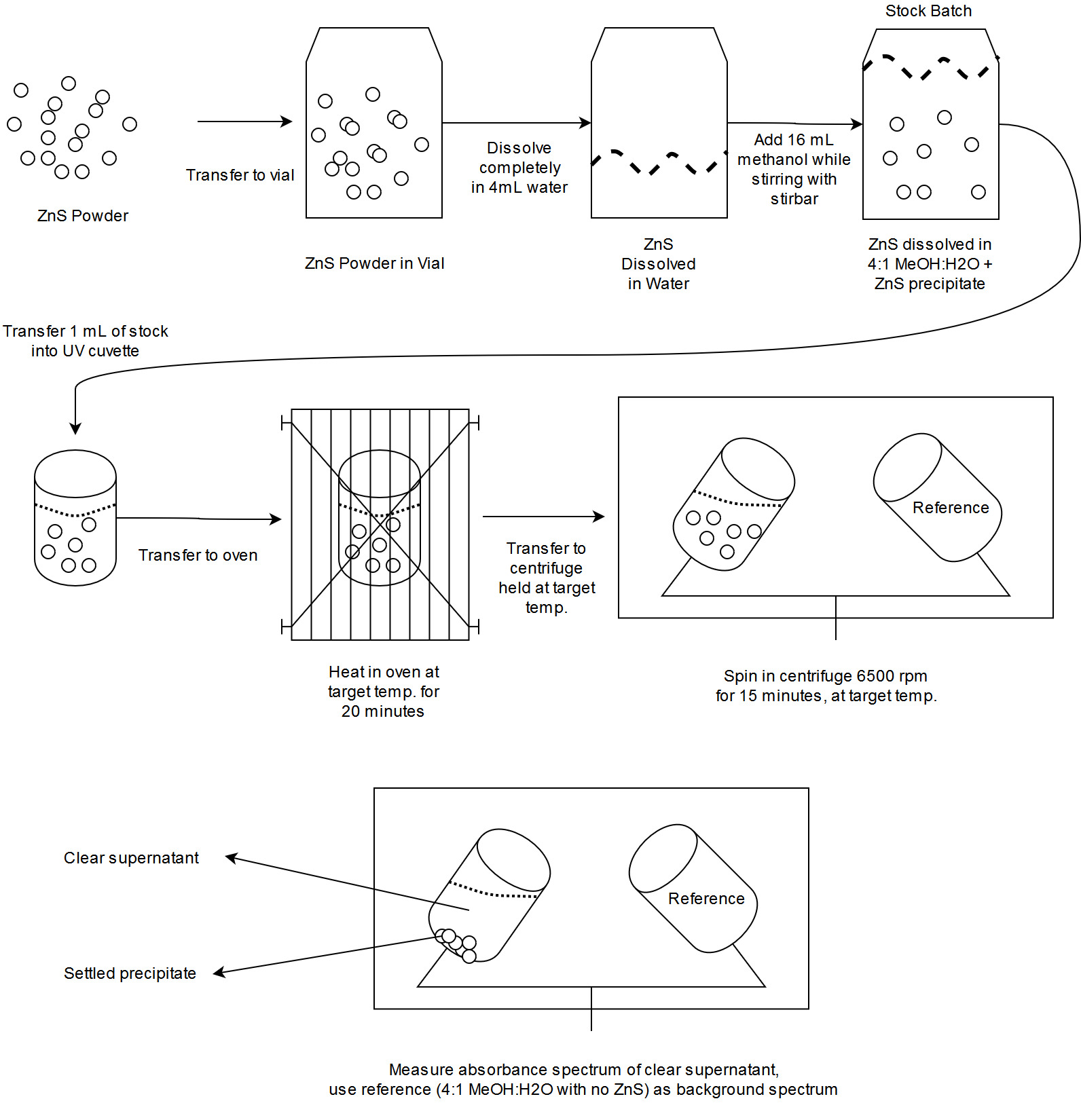
Fig. 1: Procedure for measuring absorbance of MeOH+H2O+ZnS solutions.
One such set of absorbance spectra is shown in Fig. 2.
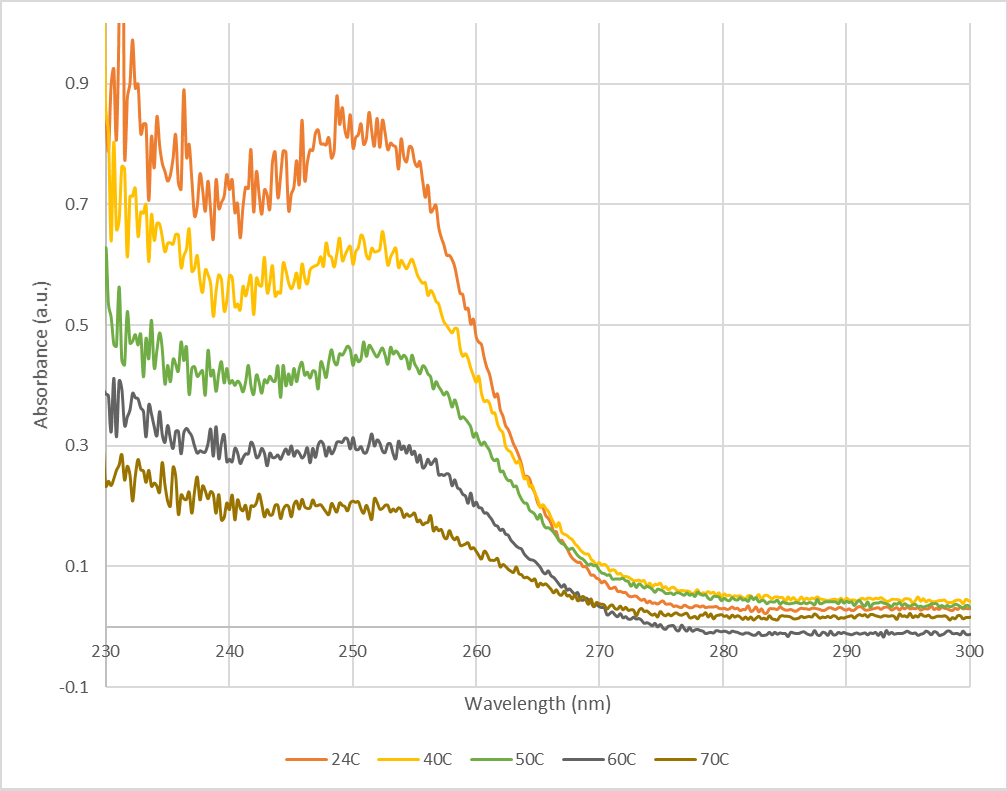
Fig. 2: Normalized absorbance spectra of ZnS NPs dissolved in 16mL MeOH + 4mL H2O at 24C (orange), 40C (yellow), 50C (green), 60C (gray), and 70C (brown).
We find that the nanoparticles are less soluble at higher temperatures, evidenced by the decreasing absorbance (and therefore concentration) with increasing temperature, shown in Fig. 2. Taking the absorbance of the peak, we plot the natural log of absorbance versus 1000/T (T in Kelvin) in Fig. 3.
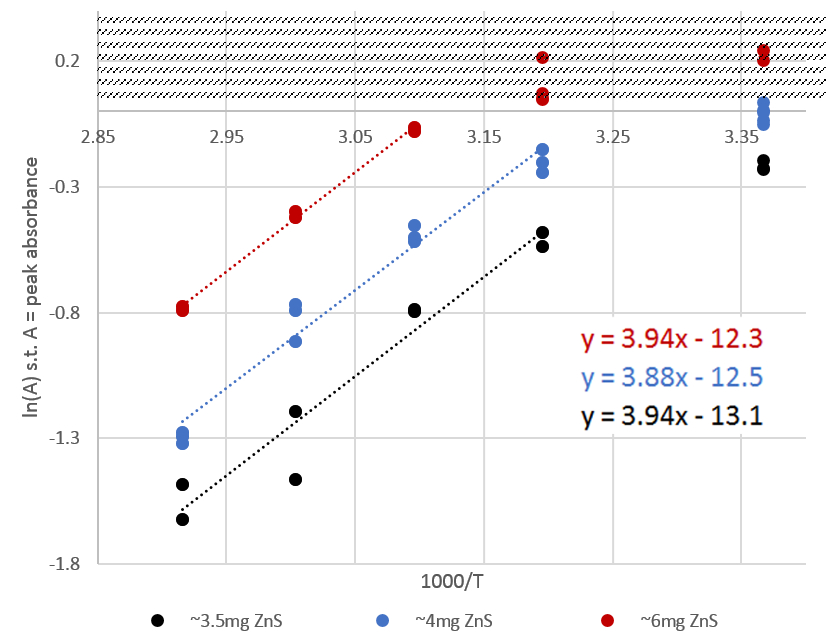
Fig. 3: Plot of ln(A) vs 1000/T for three batches of ZnS in 4:1 MeOH:H2O. The marked zone at the top of the graph indicates a measurement ceiling of our UV-Vis device where points are not reliable. Fit lines were drawn for each batch, omitting 24C data point from fit. We suspect the 24C data points are artificially low due to excess precipitation from localized methanol-rich regions during stirring and a slow redissolution rate.
By the Beer-Lambert Law, absorbance (A) is proportional to the concentration. A colligative property of dilute solutions tells us that the concentration is approximately proportional to the mole fraction. If we assume our solutions to be sufficiently dilute for this property to hold and further assume that the proportionality is constant, then we may claim that the absorbance will be (approximately) proportional to the mole fraction (x), or A=bx. Thermodynamic theory tells us ln(x) = (-∆H/RT) + c, with x the mole fraction, ∆H the enthalpy of dissolution, R the gas constant, T the temperature, and c a constant related to the activity coefficient.
Applying the proportionality discussed above, we find ln(bx) = (-∆H/RT) + c. In calculation of the slope, the proportionality constant b cancels, leaving no dependence of our slope on the value of b. Thus, if we take the average of the slopes shown in the plot to be m=4*1000 (changing from 1000/T to 1/T), we calculate an approximate enthalpy of dissolution of ∆H = -3 x 101 kJ / mol K. Notably, the ZnS NPs appear to dissolve exothermically (release heat upon dissolution), a rather uncommon property for solids.
Acknowledgments
For his mentorship and guidance throughout the project: J. Powell; Kansas State University.
For providing the nanoparticles used in this experiment: D. Segets, S. Süß; Friedrich-Alexander-Universität Erlangen-Nürnberg.