Solubility Experiments with Ligated Gold Nanoparticles
By Mo Niazi
Supervisor: Dr. Sorensen
Kansas State
University Physics Department REU Program Southern
Nazarene University


This program is funded by the National Science Foundation through grant number PHY-1157044. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.

Welcome to my webpage. This page summarizes my experience doing research for the Summer of 2013 in Dr. Sorensen’s laboratory. My project this summer was to experimentally determine the effects of temperature on Gold Nanoparticle solubility.
Project Overview
Gold Nanoparticles have many novel physical properties and applications
that are being utilized as:
-Nanoelectronic conductors for computer chips
-Photodynamic cancer therapy
-Drug delivery methods
-Chemical catalysts
-Diagnostics
To name a few…
Because of their useful and unique properties, we have chosen to study
the physics behind why these intermediate forms of matter behave the way that
they do.
Project Goals
The ultimate goal of this project is to experimentally determine how the
solubility of Gold Nanoparticles is affected by temperature.
Research
Description
This study utilized ligated Gold Nanoparticles dissolved in a non-polar
solution. This solution was then placed in a glass slip and was put through our
experimental procedure for data collection and interpretation.
Experimental Procedure 
Step One: Sonication 
We sonicate the Gold Nanoparticles into
solution.
Step Two: Equilibration
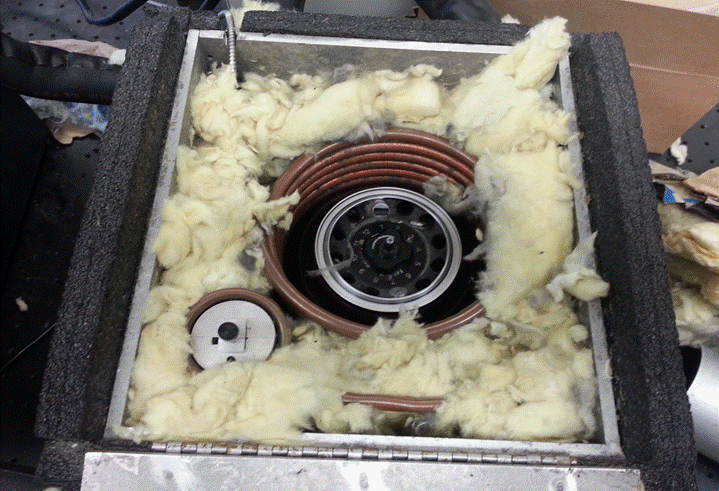
We
allow the Gold Nanoparticles to come to equilibrium with our desired
temperature for 15 minutes.
Step Three: Centrifugation

We then centrifuge the sample to bring down
any solid state Gold Nanoparticles to form a sediment
at the bottom.
Step Four: Flipping and Data Collection

The
sample is then filliped before data collection. This is done to prevent an equilibrium from forming between the solid state Gold
Nanoparticles and the
Nanoparticles already within solution.
Data
Collection Via UV-Vis Spectrophotometry

The data is then collected via UV-Vis Spectrophotometry. The data is then
analyzed and interpreted by Dr. Sorensen, Jeff Powell, And I.
Data

Absorbance vs. Wavelength Graph of seven trials overlapped.
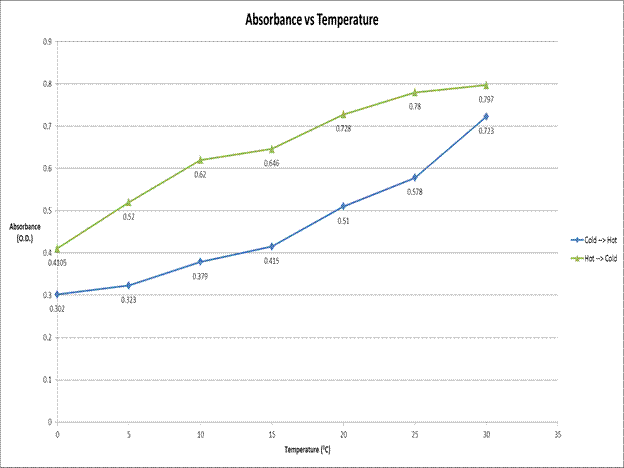
Absorbance vs. Temperature.
This enigmatic data exhibits “Hysteresis like behavior” due to the fact
that the initial temperature values influence the absorbance values of the
sample. We hypothesize that this is due to the fact that the 15 minutes that we
allowed the sample to come into equilibrium was not long enough.
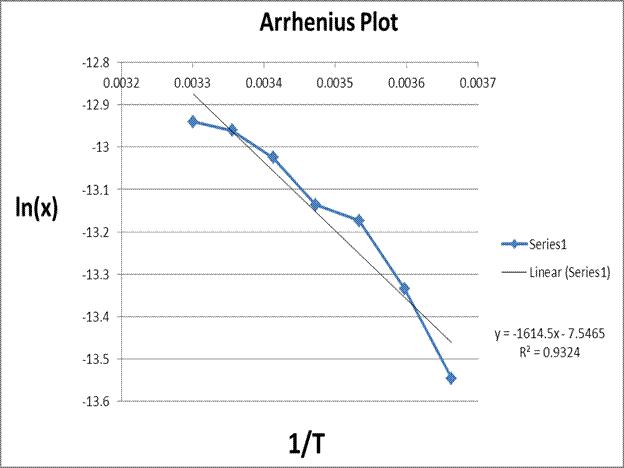
This plot allows us to calculate the change in enthalpy of dissolution
experimentally using the ideal solution equation, the slope of this graph, and
the value for the universal gas constant.
Conclusions
Upon obtaining
more data, these studies may result in a saturation curve for our Gold
Nanoparticles
The analysis
of the Arrhenius plot allowed us to experimentally determine the enthalpy of
dissolution of the gold nanoparticles in solution.
We can then
approximate the Δ Hdissolution ≈ Δ H fusion
We have yet to
make sense of the lack of equilibration found in the iterative temperature
experiments.
Final Presentation: Click here to download my presentation in PowerPoint format. This is a much more in-depth explanation of what I did this summer.
Lectures: by Dr. Weaver. I learned many new physics topics and concepts during Dr. Weaver’s talks. The subjects varied from quantum mechanics, electricity and magnetism, special and general relativity and much more. I was always excited to learn more about physics from someone of Dr. Weaver’s stature.
Ethics Class: This class brought to my attention just how cautious scientists must be with their career choices. This is due to the fact that scientists have an obligation to society to conduct research in a way that will not be harmful to society.
About Me: I grew up in Oklahoma City, Oklahoma. I am fluent in Farsi, Turkish, English, and Spanish. I am a Biochemistry “Pre-Med” major and I am currently minoring in Mathematics, Physics, and Spanish Language. I enjoy spending time with friends and family. On my spare time I enjoy learning more about science and exercising.
Here is a picture of me, Mo Niazi.
Useful Links:
Check out these useful sites:
American Physical Society Statements on Ethics
My Research group's home page: