Laser Induced Molecular Dissociation
Sam Roland
Supervisor: Itzik Ben-Itzhak
Kansas State University Physics Department REU Program, sponsored by NSF
This program is funded
by the National Science Foundation through grant number
PHY-0851599.
Welcome to my webpage! This page summarizes my
experience doing research for the Summer 2009 at Kansas State University in the
James R. Macdonald
Laboratory
Summary Statement: My research
is to understand how certain diatomic molecular ions (CO+ and NO+)
interact with a short pulse laser beam. In particular I am analyzing two possible
interactions: dissociative ionization and non-dissociative ionization.
Ionization occurs when the source ion looses an electron (ex: non-dissociative
ionization CO+ + nћω → CO++ + e-).
Dissociation occurs when the two nuclei separate (ex: CO+ +
nћω → C+ + O+ + e- ). The
experimental apparatus allows us to separate particles with different
charge-to-mass ratio in time and space, thus the two types of ionization can be
analyzed separately.
Project Goals: My aim is to count
the number of times each process occurs for a given period of time and laser
intensity. I am interested in the effect of laser intensity on the relative
frequency of dissociative and non-dissociative ionization. Furthermore I would
like to understand any observed differences between the ionization of CO+
and NO+. This project is also a demonstration of our ability to
detect both types of ionizations from a single ion source.
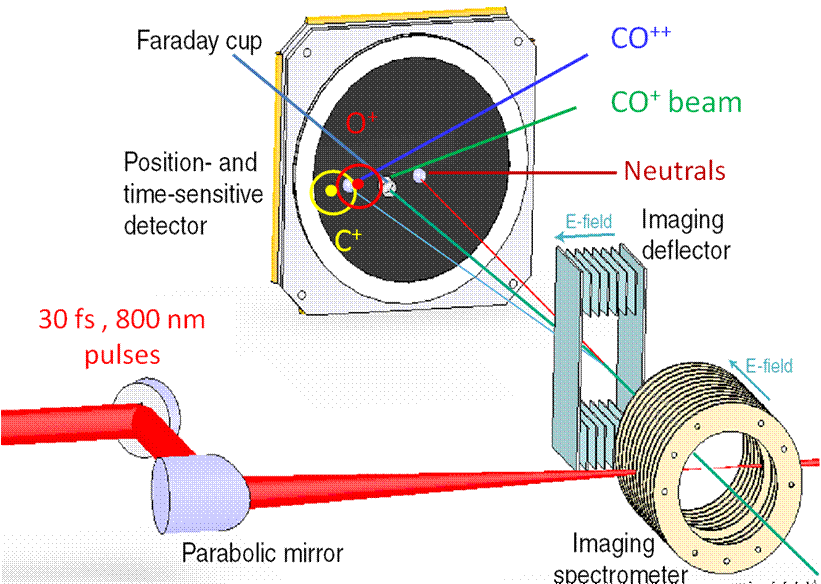
Experimental Apparatus: The laser beam is
focused by a parabolic mirror to a point inside the spectrometer.
The ion beam passes through the spectrometer where it interacts with the laser.
The resulting fragments then pass through a deflector
before reaching the particle detector. Click on the
image blow to learn more about each major component of the experimental setup.
Results and Interpretation: Analysis of data collected from seven different
laser intensities yielded the number of non-dissociative ionization events [CO++], the number of
dissociative ionization events [C++O+] and the ratio of the two events [CO++]/[C++O+] for each laser
intensity. Preliminary results show a higher rate of total ionization in CO+ than NO+. Furthermore I observed less dissociative
ionization of NO+ than CO+ but approximately
the same rate of non-dissociative ionization. These results may be accounted
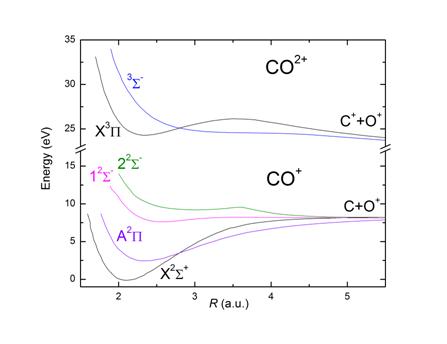
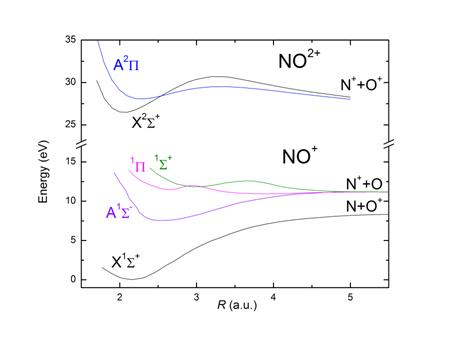
for by appealing to the potential energy curves of these various ions. The
lower total ionization rate in NO+ may be due to the
fact that the difference in potential energy between the ground states of
CO+ and CO++ is smaller than
the difference between NO+ and NO++. The smaller rate
of dissociative ionization of NO+ may be explained by the deep
potential well in the NO++ potential curves
compared to the CO++ potential curves. Furthermore I observed that the
kinetic energy release distribution of the breakup CO+ → C++O+ was qualitatively
similar the kinetic energy release distribution of the breakup CO++ → C++O+. The same observation was made for the breakup NO+ → N++O+ and NO++ → N++O+. These results suggest
that the primary ionization mechanism was direct ionization from CO+ (or NO+) states to CO++ (or NO++) states.
Potential Energy
Curves:
About Me: Rising
junior Physics and Math major at Cornell
University
Useful Links: