Measuring
the Solubility of Ligated Gold Nanoparticles
in
Hydrocarbon Solvents
by
Jeffrey Powell
Supervisor:
Dr. Chris Sorensen
Thanks
to Brandon Lohman

Special Thanks to the Kansas State University Physics Department REU Program
Who without I would not have been able to take a part in this research.
Summer Research Experience for Undergraduates 2009
Link to my Summer REU PowerPoint Presentation
Summary
Gold Nanoparticles
(AuNP) 5 nm in diameter, ligated
with various alkane thiols,
were dissolved in various alkane solvents ranging
from hexane to hexadecane and also three aromatics including toluene, para-xylene, and mesitylene. These solutions were centrifuged at 12000g
acceleration for 5 minutes to 1 hour depending on the solvent. A two-phase system forms with dissolved
monomers on top and large clusters on the bottom. The top layer of the liquid was removed and
studied because it contained pure monomers and can tell us about the solubility
of the nanoparticles in a saturated solution of
monomers. A UV-Vis Spectrophotometer was
used to find the absorbance of the solution.
The darker the liquid meant more absorbance of light and therefore more
monomers of gold nanoparticles in solution. The data from the UV-Vis was converted into
moles Au atoms per liter. Each nanoparticle system with different ligands
behaved differently in the various solvents.
We attempted to accurately find the solubility of each solvent and plot
the trend to determine the overall tendency of the AuNP
with that particular ligand.
Nanotechnology
A nanometer is one billionth of a
meter.
A carbon-carbon bond is about .15 nm
The DNA double helix diameter is 2 nm.
The smallest bacterium is 200 nm.
When comparing a nanometer to a meter,
a marble is the same as that of the earth.
Nanotechnology is
at the edge of quantum mechanics and condensed matter.
They can act and behave
as a “supermolecule” as it is on a nanometer scale.
Gravity becomes
less important and forces such as surface tension and Van der
Waals become more important.
The dramatic
increase in surface area to volume ratio alters the mechanical, thermal, and catalytic
properties of the materials.
Gold is one example of being stable
and inert in everyday quantities and sizes but becomes a potent chemical
catalyst and highly reactive at nanoscales.
Nanotechnology is
only now coming out of its infancy and we are at the tip of the iceberg. Any data or research on these nanoparticles will be helpful for the future.
The fact that these
nanoparticles can be manipulated in size and chemical
makeup makes it able to create structures with an incredible amount of new properties
and applications.
Synthesis
A
gold metal salt is reduced to slowly grow nanocrystals. The addition of a soap
causes “pods”, or micelles, to be formed where the nanocrystals
are grown. This is called the inverse
micelle method. The growth to the final
size involves diffusive interactions between the inverse micelle which contain
only a few atoms. The slow growth
determines the final size of the particles.
1) A gold salt like AuCl4- is
dissolved into a solution with a solvent like toluene.
2) A surfactant is added to the solution to promote inverse micelle
formation.
3) A stabilizing ligand is added to the
solution and is present in the inverse micelle environment.
4) A reducing agent such as NaBH4 is added to the solution to
reduce the dissolved gold ions into atoms.
5) Micellar diffusion is responsible for a
slow growth rate of particles giving rise to nanocrystalline
structures instead of disordered clusters.
6) The product of inverse micelle synthesis is digestively ripened.
Digestive Ripening
For the nanoparticles to be useful for any systematic study or size-dependent application, they must be monodisperse in size distribution. Digestive ripening is a technique used in which polydisperse ligated gold nanoparticles are heated and refluxed anaerobically in the presence of excess ligand. The mechanism is poorly understood but involves the nanoparticles trading their constituent atoms or groups of atoms back and forth until and equilibrium size is reached. A driving force for this favored equilibrium size can be a consideration of the competition between the surface energies of the particles favoring large size and the interaction of the ligand with the metal surfaces favoring small sizes.
Transmission
Electron Micrograph Pictures Post Digestive Ripening Pre Digestive Ripening
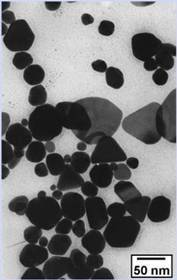
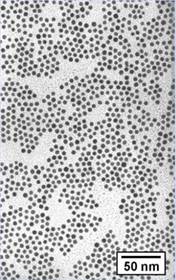
This
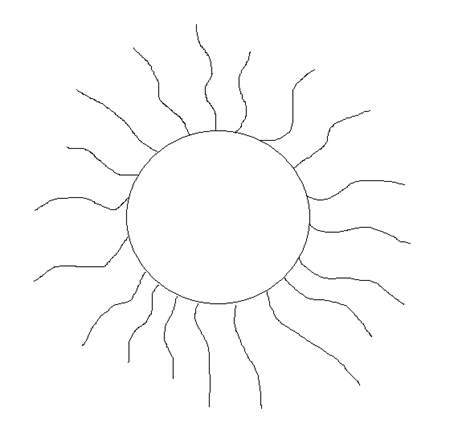
is a rough sketch of what a thiol ligated nanoparticle would
look like. The center sphere is the
gold nanoparticle while the floppy “spaghetti” is
the thiols attached to the surface of the gold
through covalent bonds. A typical dodecanethiolated
gold nanoparticle has the chemical formula of Au3850(C12SH)350.

Experimental
Our
experiment was to test the solubility of these alkanethiolated
gold nanoparticles in various solvents. We took dry AuNP
and used alkane and aromatic solvents to dissolve the
particles. Dry AuNP
were added to 300 microliters of solvent and sonicated to dissolve all particles. This was done until no more gold would
dissolve in the solvent and a precipitate was seen. This meant that there was an
equilibrium of gold in solution and precipitated gold. These samples were then spun in a centrifuge
at 12000g acceleration until a distinct two-phase system appeared. This high acceleration pushed all of the
clusters to the bottom and left the monomers in solution. This supernatant of monomers in solution was
removed carefully. The supernatant was
then taken to the UV-Vis spectrophotometer where its absorption was measured
between 400 nm and 600 nm. Below is a
plot of the data gathered from the UV-Vis.
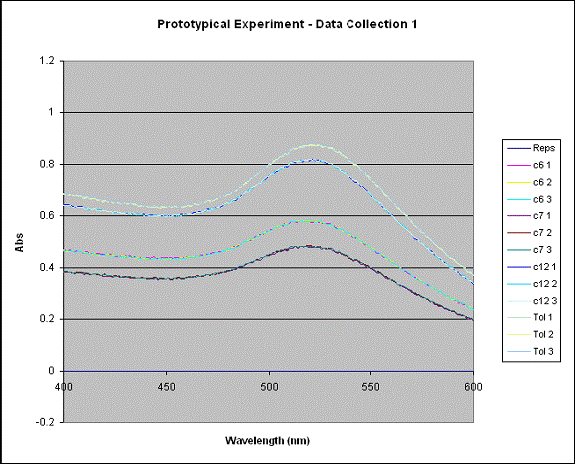
The peak that is seen is the plasmon peak. The plasmon is a quantum of plasma oscillation of the free
electron gas at the surface of the gold particle. The absorbance at this peak was what was used
to find the concentration of gold atoms in this solvent. Using Beer’s Law, the absorbance is converted
into concentration in moles of gold atoms per liter solvent. This procedure was done using octanethiolated AuNP, decanethiolated AuNP, dodecanethiolated AuNP, and hexadecanethiolated AuNP. The results are shown below.
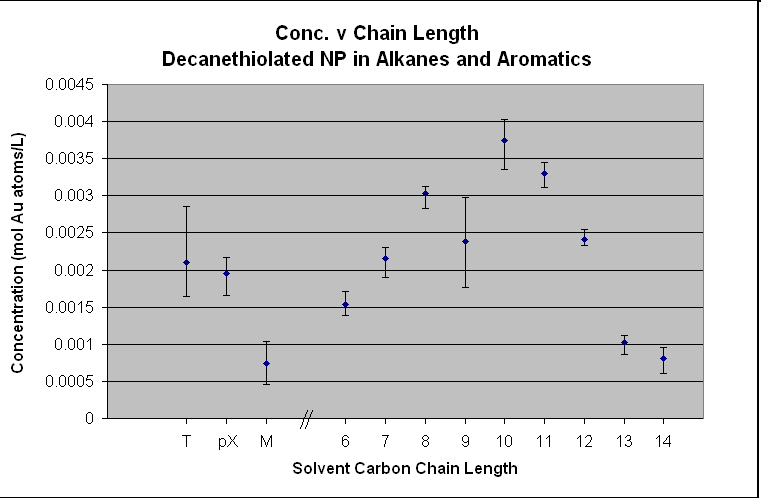
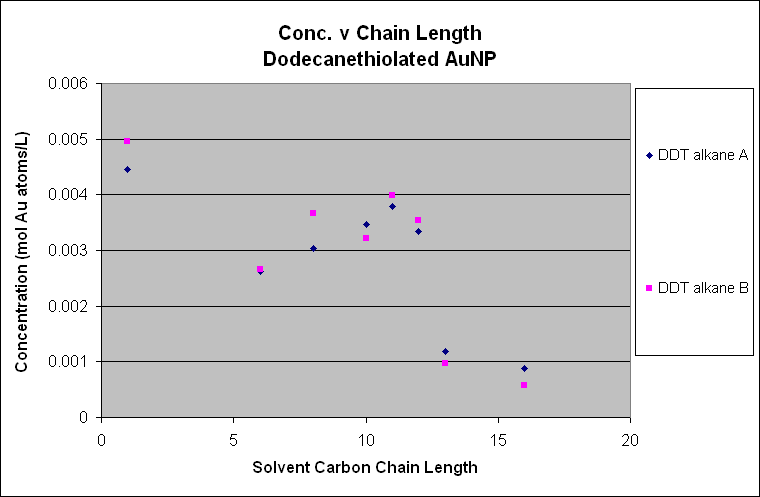
In these two plots, it is easy to see
the trend among the alkane solvents. In the decanethiolated
AuNP, the solubility increases from hexane to decane where it peaks.
The solubility then drops in the higher chain alkanes. The same is seen in the dodecanethiolated
AuNP except the peak concentration is seen around dodecane. The trend
seems to suggest that the ligated AuNP
follows a “like dissolves like” conclusion.
The ligands on the gold sphere are attached by
a sulfur atom. This sulfur atom is
“busy” and occupied with bonding to the gold.
The “tail” of carbon atoms left on the thiol
is left to move around and “flop”. When
dissolved in a solvent, an alkane similar in length
to the ligand will be more likely to draw up into
solution the entire AuNP. Dodecanethiolated AuNP will be most soluble in dodecane,
as seen in the plot.
In the decanethiolated
AuNP plot, there is a
“hiccup” at nonane where the solubility decreases
after octane instead of following the trend upwards toward decane. This was acknowledged during our experiment
that something strange and as of now unexplainable. We are confident it is a true point because
of our experimental method and techniques.
The “like dissolves like” trend is
seen in the example of how a small amount of ethanol can be dissolved in water
but an infinite amount of water can be dissolved in water. The “sameness” of the nanoparticle
and the solvent leads to better solubility.
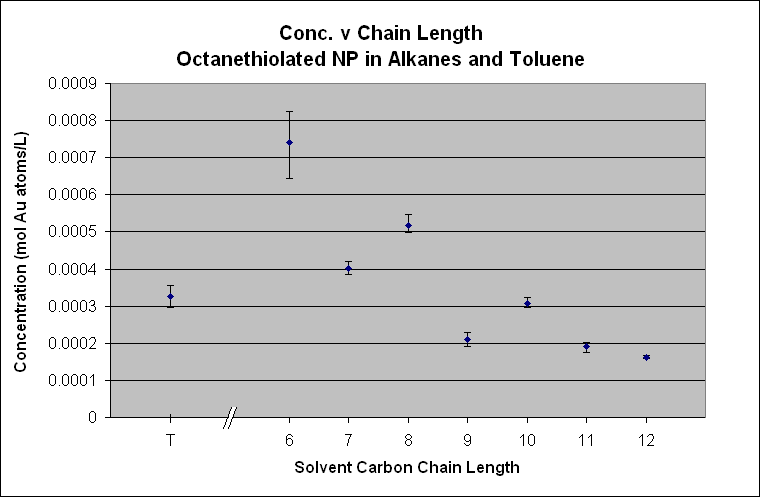
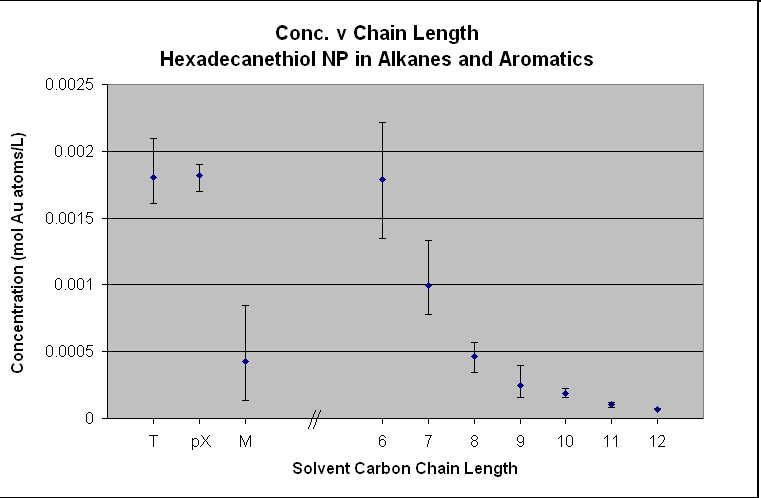
The
two previous graphs of the octanethiolated AuNP and the hexadecanethiolated AuNP show a different story. They obviously do not follow the “like
dissolves like” hypothesis as the octanethiolated AuNP does not have a peak at octane and the hexadecanethiolated AuNP does not
have any solubility after dodecane. If the magnitude of the concentration is
compared to that of the decanethiolated AuNP and dodecanethiolated AuNP the C8 and C16 particles are an
order of magnitude less. The octanethiolated AuNP also have an
obvious stair-stepping with an odd-even functionality. These two plots do not support the hypothesis
of “like dissolves like” and some unknown forces are acting on these particles
that dramatically affect their solubility in these solvents.
Conclusion
This is the first example of a
suspension of particles acting as a thermally reversible solution. These nanoparticles
are acting as molecules in solution and are capable of changing phases with no
change in entropy. This is unique
because of the sizes of these “supermolecules.” They are not just a colloid with particles
suspended in liquid but actual “molecules” with physical properties directly
affecting the entire solution.
Future work on this project would deal
with finding why the intermolecular forces decide the solubility they way that
they do. This would answer the question
of why changing the ligand length can increase or
decrease the solubility of the nanoparticles.
Acknowledgements
NSF NIRT
CTS0609318
This work
was partially funded under NSF grant number PHY-0851599.
Any opinions, findings, and conclusions or
recommendations expressed in this material
are those of the author(s) and do not
necessarily reflect the
views of the National Science Foundation.
Dr. Chris
Sorensen
Brandon Lohman
Kansas
State Physics and Chemistry Departments
Kansas
State Biochemistry Department and Dr. John Tomich
About Me
My
name is Jeffrey Powell and I graduated May 2010 from Benedictine College in Atchison,
Kansas. I am double majored in Physics
and Chemistry with a minor in Mathematics.
I am currently in physics graduate school at Kansas State University. This project was completed with the help of
Brandon Lohman under the guidance of Dr. Sorensen
during my 10 week summer REU internship with the Kansas State Physics
Department. The project was started May
2009 and ended July 2009.
