 |
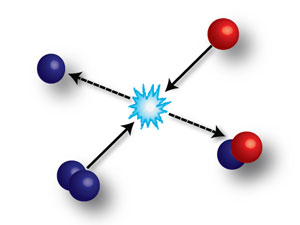
When a molecule (two blue spheres) collides with an atom (single red sphere), an atom can be exchanged. A new molecule is produced (red and blue spheres) and an atom (single blue sphere) is released. In the experiment performed in Innsbruck this process is observed at temperatures of less than one millionth above the absolute zero. The exchange is completely determined by the quantum nature of the matter and can be controlled by a magnetic field. (Caption: Eureka Alert; Image: IQOQI) |