Attosecond pulses probing electron dynamics: The natural time scale for the electronic motion in atoms and molecules is in the order of 100 attoseconds (1as=10-18 seconds) or less. With the emergence of light pulses of duration of hundreds of attoseconds or less, it becomes possible to ask whether one can interrogate how the motion of the electrons in an atom is affected by each other. The simplest system that such an effect can be probed is the helium atom.
Traditionally the motion of the two electrons in helium is perceived as independent. This is not true since the two electrons interact with each other via the Coulomb force and this effect is particularly large when they are close to each other, or when both electrons are relatively slow. The latter occurs when helium atom is in doubly excited states. In this case, the two electrons tend to move jointly, and executing rotational, stretching and bending vibrational motions similar to the motion of atoms in a molecule. The periods of these normal modes are in the order of hundreds of attoseconds or a few femtoseconds.
Here we show how these different modes appear when wave packets of a helium atom are created.
(1) A simple wave packet made of 1s1s 1S +1s2s 1S at t=0 will oscillate with a period depending on their energy difference. In this case it is the distance of the outer electron from the nucleus that is changing in time. See the movie clip below.
(2) If a wave packet is made of two doubly excited states,
say 2s2 1S +2p2 1S at t=0, then the
oscillation is the bending vibration. In the movie clip below, it is a plot of
the density distribution of the two electrons in the plane of (![]() ) where the first angle measures the relative distances of the
two electrons from the nucleus,
) where the first angle measures the relative distances of the
two electrons from the nucleus, ![]() and
and ![]() is the angle between the two electrons measured from the
nucleus. The movie shows that for this wave packet the two electrons execute
bending vibrational motion.
is the angle between the two electrons measured from the
nucleus. The movie shows that for this wave packet the two electrons execute
bending vibrational motion.
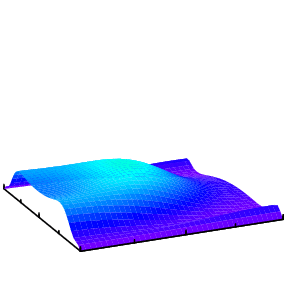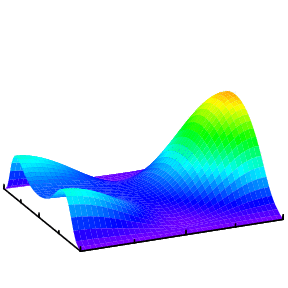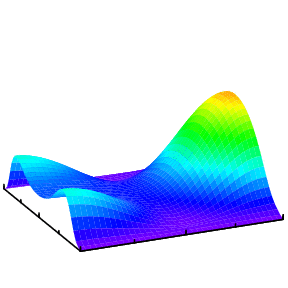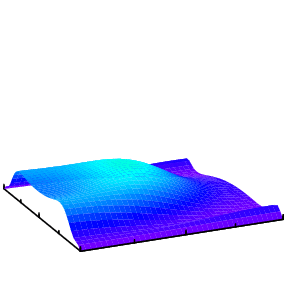
In general, when a helium atom is excited by a light pulse, different modes will be excited and which mode is observed would depend on the property of the probe pulse. One way is to doubly ionize the helium and measure the momentum distributions of the two electrons. The analysis of such data to extract the initial state information is more challenging and is a subject of our research.