Nucleation!!!!!!!!!
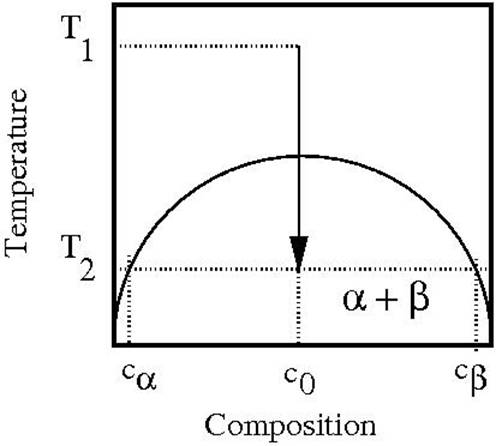
At a given temperature, there is a volume
fraction that is ideal for the growth of nucleating clusters in a system. These conditions appear near the boundary of the
phase diagram that exists if you plot temperature as a function of
concentration for a given system (figure 1).
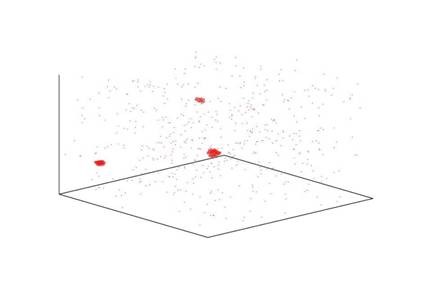
Figure 2 shows the morphology of three nucleating clusters at a
concentration k=8, T=.15kt, number of monomers=1000, and box size =128 (volume
fraction=.00025). Nucleating clusters
grow uniformly and have a compact structure, resembling that of a sphere with a
fractal dimension nearing the value of 3.
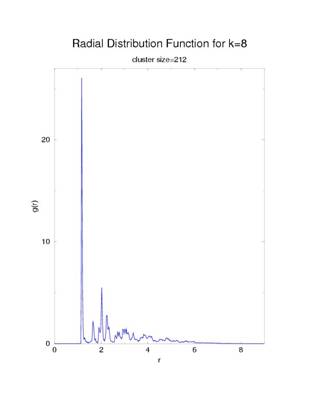
To study the structure of the nucleating clusters, I plotted the radial
distribution function of the largest cluster in a system as a function of
radial distance (figure 3). To see an
image of this see the section on Lysozyme.
The slightly sharp peaks are indicative of a more tightly bound
structure due to the large probability that a monomer will be at a given
position. In contrast a liquid would
have very broad distribution with dull peaks due to the particles ability to
move around more freely.